MANUFACTURING INTERNAL CONTROL (AFB) SMEARS BY PHAS AND BAS TREATED SPUTUM SAMPLES
SALT Journal of Scientific Research in Healthcare
Volume 1, Issue 2, Page 38-41, Published on 04th December 2021
https://doi.org/10.56735/saltjsrh.ms2101023841
Research Article
*SHERAFIN JANCY VINCY AND M CHANDRASEKAR
Sree Balaji College of Physiotherapy,
Bharath Institute of Higher Education and Research, Chennai, India.
*Corresponding Author: Dr Sherafin Jancy Vincy, Professor, Sree Balaji College of Physiotherapy, Bharath Institute of Higher Education and Research, Chennai, India
ORCID ID: 0000-0001-5196-4027
Email: drsjvincy@gmail.com
ABSTRACT
Objective: Internal control smears were prepared using phenol ammonium sulfate (PhAS) and bleach ammonium sulfate (BAS) methods. Methods: A complete of 150 smears were prepared, 80 smears were stained, and two different technologists validated 60 smears. Results: Consistency was found to be true when compared with the quality consistency table for all grades in both methods, and M±2SD was within the boundaries. Conclusion: This study suggests that PhAS and BAS are alternate concentration methods for the preparation of internal control smears.
Keywords: Internal control, PhAS, BAS, sedimentation, grade suspension
1.0 INTRODUCTION
Tuberculosis (TB) is caused by mycobacterium tuberculosis, a prototypical airborne pathogen1, infecting one-third of the planet population2. TB is among the highest ten causes of death worldwide3,4. Sputum cytosmear microscopy is that the foremost widely available diagnostic assay for consumption in countries with a high burden of the disease5, 6.
Internal control or proficiency testing (PT) consists of staining and reading centrally prepared slides with known numbers of acid-fast bacilli (AFB)7. In national-level laboratories, sputum is concentrated with sodium hydroxide (NaOH), and N-acetyl L-cysteine (NALC) methods 8, 9, 10 and internal control (IC) slides were prepared as per the guideline of the World Health Organization (WHO) and also the International Union Against Tuberculosis and Lung Disease (IUATLD) guidelines. Other concentration methods include phenol ammonium sulfate (PhAS)11,12 and bleach ammonium sulfate (BAS)13 methods. So on attain the specified technical quality with the preparation of smears, standard techniques for the digestion of sputum are needed. The quality sputum concentration method for manufacturing smears in PT improves the standard of slides and remains a priority for practical training. Hence, we compared phenol ammonium sulfate (PhAS) with bleach ammonium sulfate (BAS) sputum digestion methods to prepare PT smears.
MATERIALS AND METHODS
PhAS reagent was prepared by dissolving 5g of phenol and 4g of ammonium sulfate in 100 ml of distilled water11. Preparation of 5% BAS reagent involved, dissolving of 5g bleaching powder and 4g ammonium sulfate in 100 ml of distilled water 13,14. Sputum samples were collected from the Institute of Thoracic Medicine and Tuberculosis Hospital, Chennai. Negative samples from different non-positive patients with 20 or more white blood cells per field were collected, and 3+ positive samples with a bacillary load of roughly 50 AFB per field were collected. Initially, direct smears were taken from sputum samples and stained with ziehl neelsen (ZN) stain. The number of cells and bacilli in 100 fields were counted and recorded in standardized forms containing 100 boxes for both negative and positive samples simultaneously. The pooled positive and negative sputum samples were aliquoted into two portions that are approximately equal in volume. The two portions were randomly allocated, first to PhAS method and second to BAS method.
PhAS positive stock: A sample of three ml positive 3+grade sputum was taken in a McCarteny bottle, and an equal volume of PhAS reagent was added. After half-hour minutes, the supernatant was discarded, and also the deposits were mixed well. This residue solution was considered as PhAS positive stock solution.
BAS positive stock: A sample of three ml of sputum was taken to which an equal amount of reagent was added, it was incubated overnight to concentrate the bacilli, and also the supernatant was discarded13. The sputum deposit was vortexed for about 5 minutes to urge BAS positive stock solution.
Initial smear was taken from the deposit of PhAS and BAS, respectively, and later smears were taken from PhAS and BAS positive stock solution, respectively. These smears were stained by ZN stain15 and validated for 100 boxes to assess the typical bacilli/ field, which was found to be 70 and 80 bacilli/ field for PhAS and BAS method, respectively.
Negative stock solution preparation involved the addition of 10% formalin per ml of negative sputum, and then it was appropriately mixed by the vortexer mixer. Negative grade suspension smears were prepared directly from the negative stock. So on getting positive (Scanty, 1+, 2+, 3+) grade suspension, the stock solution of positive AFB sputum prepared by both PhAS and BAS sedimentation was diluted with the negative stock solution, respectively. For calculation of the dilution factor, the subsequent formula was used: N = (DC/AC) X A, where N is the number of drops of positive sputum to be added, DC is the desired AFB concentration, AC is the actual AFB concentration, and A is that the number of drops during a given volume. A Pasteur pipette was used to grasp the number of drops per ml. AC was obtained in an exceedingly smear made with two drops of every grade suspension prepared16.
Each grade suspension prepared by the above-described procedures was vortexed for five minutes, and twenty-five slides were prepared from each grade (3+, 2+, 1+, Scanty, and Negative) suspension. From 25 slides randomly, eight slides were selected and stained by ZN stain. Then from eight stained slides, six slides were randomly selected and validated17, 20. Data were entered and processed using Microsoft Excel. The mean (M), standard deviation (SD), and consistency (M±2SD) were calculated to assess the equality of PhAS method with BAS method for manufacturing internal control slides.
RESULTS AND DISCUSSION
Tables 1 and Table 2 shows the results of validation of manufacturing internal control slides by PhAS and BAS methods, respectively. Table 1 shows SD for 3+, 2+, 1+, scanty and negative as 9, 1, 10, 1, 0 respectively. Table 2 shows SD for 3+, 2+, 1+, scanty and negative as 5, 1, 14, 1, 0 respectively. M±2SD were found to be within the boundaries regardless of the used methods (PhAS and BAS). Consistency was true for both methods for 3+, 2+, 1+, scanty and negative grades.
In 2010, of 36 countries with the best burden of tuberculosis and multidrug-resistant tuberculosis, 20 countries had but one laboratory capable of doing culture for each 5 million people, over 80% of the estimated 8.8 million people with incident tuberculosis board high-burden countries. As per the estimates of WHO, 10% of patients with tuberculosis in low-resource settings had their disease proven with culture or biological science approaches18. Therefore, assuming a conservative estimate of 20% default, quite 1.5 million people will have a missed or delayed diagnosis per annum. Insight of the vast numbers of patients involved, incremental improvements in smear microscopy will cause substantial increases within the numbers of patients detected at little or no cost. However, nowadays, most patients with tuberculosis have access to only smear microscopy.
The procedure recommended by WHO for manufacturing slides for internal control was to process the sputum with NaOH and NALC19. The present study involved sputum digestion by PhAS and BAS methods. The manufactured smears by the PhAS and BAS methods were screened by two readers; however, the smears randomly coded were specified. The reader who reads the slide was unable to spot whether the slides were processed by PhAS or BAS method. Because the PhAS and BAS methods are distinct in appearance, it absolutely was impossible to blind the reader from the sort smear. Smears prepared by PhAS and BAS deposits were found to be intact. Probably, ammonium sulfate precipitated the mucus component of the sputum, allowing firm fixation of the smears on the slides. PhAS sediment smear was reported to be easy to read, with well-defined margins and with distinct AFB against a blue cell background. Other advantages of this method are that phenol is inexpensive, stable at room temperature, and maybe prepared at reference laboratories and supplied to peripheral health centers11. The most significant limitation of the BAS method is that it necessitates overnight sedimentation, which delays the time interval for internal control smears.
CONCLUSION
This study suggests that PhAS and BAS methods may be used as alternate methods for internal control smear preparation together with conventional NaOH and NALC methods. In National level laboratories where large numbers of technicians are trained, the PhAS and BAS method can substantially increase the efficiency of preparing internal control smears. However, evaluation of cold staining on the sputum concentration method is desirable.
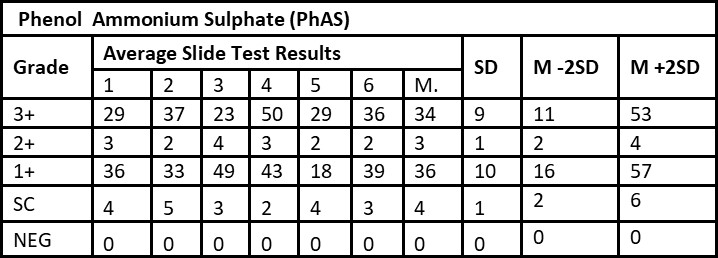
Smear results: 3+: Over 10 AFB per oil immersion field in a minimum of 20 fields; 2+: 1 to 10 AFB per oil immersion field in a minimum of 50 fields; 1+: 10 to 99 AFB per 100 oil immersion fields; Scanty: 1 to 9 AFB per 100 oil immersion fields. M: Mean, SD: Standard Deviation, Cons: Consistency
REFERENCES
- Morrison J, Pai M, Hopewell PC. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2008 Jun;8(6):359-68. https://doi.org/10.1016/S1473-3099(08)70071-9. Epub 2008 Apr 29. PMID: 18450516.
- Drobniewski FA, Caws M, Gibson A, Young D. Modern laboratory diagnosis of tuberculosis. Lancet Infect Dis. 2003 Mar;3(3):141-7. https://doi.org/10.1016/s1473-3099(03)00544-9. PMID: 12614730.
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006 May 27;367(9524):1747-57. https://doi.org/10.1016/S0140-6736(06)68770-9. PMID: 16731270.
- Dye C, Bassili A, Bierrenbach AL, Broekmans JF, Chadha VK, Glaziou P, Gopi PG, Hosseini M, Kim SJ, Manissero D, Onozaki I, Rieder HL, Scheele S, van Leth F, van der Werf M, Williams BG. Measuring tuberculosis burden, trends, and the impact of control programmes. Lancet Infect Dis. 2008 Apr;8(4):233-43. https://doi.org/10.1016/S1473-3099(07)70291-8. Epub 2008 Jan 16. PMID: 18201929.
- Kirwan DE, Gilman RH. Same-day diagnosis and treatment of tuberculosis. Lancet Infect Dis. 2013 Feb;13(2):102-4. https://doi.org/10.1016/S1473-3099(12)70270-0. Epub 2012 Oct 23. PMID: 23099182.
- Davis JL, Cattamanchi A, Cuevas LE, Hopewell PC, Steingart KR. Diagnostic accuracy of same-day microscopy versus standard microscopy for pulmonary tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2013 Feb;13(2):147-54. https://doi.org/10.1016/S1473-3099(12)70232-3. Epub 2012 Oct 23. PMID: 23099183; PMCID: PMC3836432.
- Martinez-Guarneros A, Balandrano-Campos S, Solano-Ceh MA, Gonzalez-Dominguez F, Lipman HB, Ridderhof JC, Flisser A. Implementation of proficiency testing in conjunction with a rechecking system for external quality assurance in tuberculosis laboratories in Mexico. Int J Tuberc Lung Dis. 2003 Jun;7(6):516-21. PMID: 12797692.
- Iseman MD. A Clinician’s guide to tuberculosis. Philadelphia: Lippincott, Williams and Wilkins, 2000; 29.
- Steingart KR, Ng V, Henry M, Hopewell PC, Ramsay A, Cunningham J, Urbanczik R, Perkins MD, Aziz MA, Pai M. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis. 2006 Oct;6(10):664-74. https://doi.org/10.1016/S1473-3099(06)70602-8. PMID: 17008175.
- Kent PT and Kubica GP. Public health mycobacteriology: a guide for the level III laboratory. Atlanta: Centers for Disease Control, 1985.
- Selvakumar N, Rahman F, Garg R, Rajasekaran S, Mohan NS, Thyagarajan K, Sundaram V, Santha T, Frieden TR, Narayanan PR. Evaluation of the phenol ammonium sulfate sedimentation smear microscopy method for diagnosis of pulmonary tuberculosis. J Clin Microbiol. 2002 Aug;40(8):3017-20. https://doi.org/10.1128/JCM.40.8.3017-3020.2002. PMID: 12149368; PMCID: PMC120642.
- Sherafin Jancy Vincy, Sheela S and Venkatesan P. Evaluation of preparation of quality control (AFB) smears by Phenol ammonium sulphate method. J Appl Zool Res. 2007; 18(2):162-165.
- Chandrasekar M, Sherafin Jancy Vincy and Venkatesan P. Evaluation of Bleach ammonium sulphate sedimentation method for preparation of acid fast bacilli smears for panel testing and quality control. Asian J Microbiol Biotech Env Sc. 2008; 10(2): 393-398.
- Sherafin Jancy Vincy, Vincent S, Prabakaran M, Chandrasekar M, John Regulus Gunanithy, Rajalakshmi R and Bashir Ali Siyad. Comparative studies on preparation of Panel Test (AFB) Smears by BAS and NALC Treated Sputum samples. Intern. J Current Res. 2013; 5(1).
- Sherafin Jancy Vincy, Chandrasekar M and Venkatesan P. Evaluation of rapid AFB cold (RAC) staining method for sputum smears treated with bleach ammonium sulphate (BAS). J P Appl Micbiol. 2008; 02(02): 431-435.
- Perkins MD. New diagnostic tools for tuberculosis. Int J Tuberc Lung Dis. 2000 Dec;4 (12 Suppl 2):S182-8. PMID: 11144551.
- Chandrasekar M and Venkatesan P. Evaluation of dilution method for preparation of panel test smears. J Appl Zool Res.2007;19(1): 90-92.
- WHO. Global tuberculosis control. Geneva: World Health Organization, 2011.
- Aziz M A, Ba F and Beex-Bleumink M. External quality assessment for AFB smear microscopy. PHL, CDC, IUATLD, KNCV, RIT. Washington, DC: Association of Public Health Laboratories. 2002; 1-111.
- Selvakumar N, Ravikumar D, Sivagamasundari S, Gopi PG, Narayanan PR. A novel method of staining acid-fast bacilli in sputum containers. Indian J Med Res. 2006 Jun;123(6):776-80. PMID: 16885599.
ARTICLE TYPE: Research Article;
ORCID ID: Open Researcher and Contributor Identifier (ORCID) ID of corresponding author: https://orcid.org/0000-0001-5196-4027;
ETHICAL: Permission from Institutional Ethical Committee;
ACKNOWLEDGEMENT: The authors are thankful to Loyola College (Autonomous), Chennai, for providing the necessary facilities for this investigation. We gratefully acknowledge encouragement from Dr. N. Selvakumar, Former Deputy Director, National Institute of Tuberculosis Research (ICMR), Chennai, for his critical inputs and The Former Director, Institute of Thoracic Medicine and Tuberculosis Hospital, Chennai, for providing sputum samples and necessary facilities.;
FINANCIAL DISCLOSURE: The authors declare that there was no financial aid received.;
CONFLICT OF INTEREST: No conflict of interest associated with this research work.;
AUTHORS CONTRIBUTION: M.C., performed the slide preparation and staining, S.J.V., Designed research. Both authors discussed the results and prepared manuscript.;
CORRESPONDING AUTHOR AFFILIATIONS: Dr Sherafin Jancy Vincy, Professor, Sree Balaji College of Physiotherapy, Bharath Institute of Higher Education and Research, Chennai, India;
CORRESPONDING AUTHOR EMAIL: drsjvincy@gmail.com;
ARTICLE CITATION: Vincy SJ, Chandrasekar M.Manufacturing internal control (AFB) smears by PhAS and BAS treated sputum samples. SALT J Sci Res Healthc. 2021 December 04; 1(2): 38-41.
PUBLISHER’S NOTE: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.




© Sherafin Jancy Vincy and M Chandrasekar.
Originally published in the SALT Journal of Scientific Research in Healthcare (https://saltjsrh.in/), 04.12.2021.
This is an open-access article distributed under the terms of the Creative Commons License (https://creativecommons.org/licenses/by-nc-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the SALT Journal of Scientific Research in Healthcare (https://saltjsrh.in/), is properly cited. The complete bibliographic information, a link to the original publication on https://saltjsrh.in/, as well as this copyright and license information must be included.
