EPIDEMIOLOGY OF CHIKUNGUNYA IN INDIA- A SHORT REVIEW
SALT Journal of Scientific Research in Healthcare
Volume 2, Issue 1, Page 40-45, Published on 04th March 2022
https://doi.org/10.56735/saltjsrh.ms2202014045
Review Article
MOHAMMADAMANBAHADURI1, *LOUIS COJANDARAJ2, JUHI KATARIA3
1 Department of Microbiology, University of Kabul, Afghanistan
2 Department of Medical Laboratory Sciences, Lovely Professional University,Phagwara, Punjab-144411, India
3 Department of Medical Laboratory Science, Khalsa College of Pharmacy and Technology, Amristar, Punjab, India
ABSTRACT
Chikungunya is a viral infection triggered by CHIKV, which belongs to the alphavirus family and is transferred by the bites of infected Aedes mosquitoes both A. aegypti and A.albopictus to humans. During the post-storm season, the transmission of the infection increases due to the increase in the population of the mosquito. It demonstrates the same pathogenicity as dengue fever. The symptoms initiate within 4 and 7 days of the patient being chomped by the CHIKV- infected vector. Symptoms include high fever (40°C/104°F), chills, cerebral pain, regurgitation, joint agony (lower back, lower leg, ankles, wrists, or phalanges), muscle torment, nausea, fatigue rash, and arthralgia. It is thought that the important source, or stockpile, of mosquito Chikungunya infection, is Homo sapiens. In researching the data sets from 1948 to 2017 (69 years), we noted that more cases were recorded for Chikungunya especially from the eastern and western parts of India during the period 1982-2017 in contrast to 1948 -1981 when there was an unremitting increase in the standard temperature. Chikungunya instances began to spike during 1982-2016 when ordinary temperatures had risen to just 29°C. At the temperature (27- 34°C), A. aegypti and A. albopictus, the basic bearers, indicated a greater gnawing frequency, and the most amazing pervasive rodent of chikungunya instances (83.6 per million population) was accounted for in 2006-2017. This review was intended to determine the chikungunya’s status in India and to comprehend the risk factors associated with the growing incidence of chikungunya.
Keywords: Chikungunya, CHIKV, Epidemiology, Makonde plateau, Aedes aegyti, Aedes albopictus
INTRODUCTION
India is one of the nations in the globe known for its population and geographic diversity that affects the habitat of various species of mosquitoes related to the transitions of various vector-borne illnesses, and chikungunya is one of those vector-borne diseases transited through CHIKV which targets human beings through the non-target organisms1. The symptoms include acute and chronic arthralgia, rash, cephalgia headache, and hyperpyrexia. During the initial stages, the pathogenicity of Chikungunya resembles that of dengue fever.2 Mostly this infection is not life-threatening, but some symptoms such as arthralgia may last with the patient for a long time and require several months to reconstruct. The people exposed to CHIKV infections for the first time develop antibodies against CHIKV which provides the least exposure to the recurrent CHIKV infections.3 The rate of rehashed and prolonged illness of arthritis causes a critical disability in one percent of the CHIKV influenced population and can significantly affect the social economy of the nation.4 Chikungunya fever is triggered by CHIKV, a part of the alphavirus genes belonging to the toga-Viridae family that spread through the Aedes aegypti bites. It originated from the word “Makonde” which means “that which bends up”, an arthritic sign of disease. In 1952 during the epidemic outbreak in Makonde plateau, Tanganyika and Mozambique CHIKV was first identified from samples of humans and mosquitoes. CHIKV genome includes 11.8 Kb of single-stranded RNA molecule.5 A genetic study of shift mutated genes (A226V to E1 protein) of this virus, was observed in the progression of epidemic instances of the Reunion islands was absent in all the recorded Indian instances up to 2007, but in 2007 only one case was reported on this mutated genes.6 This review was intended to determine the chikungunya’s current status in India and to comprehend the risk factors associated with the growing incidence of chikungunya.
CHIKUNGUNYA VIRUS
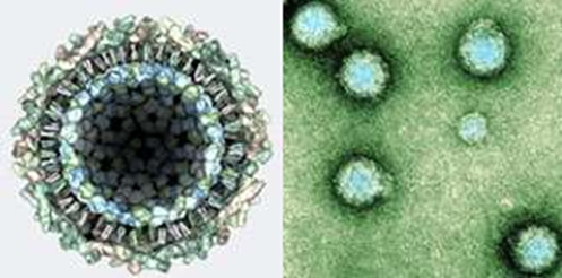
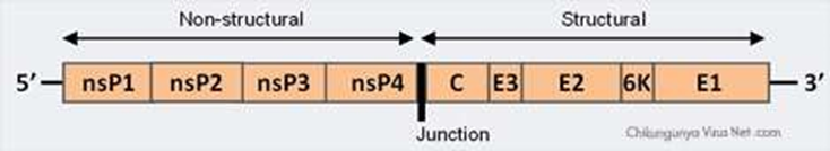
Chikungunya is triggered by the arthropod-borne disease (arbovirus) Chikungunya virus (CHIKV) belonging to the Togaviridae family of the Alphavirus genus. Nearly about 29 distinct kinds of alphaviruses are reported to date. These arbovirus species were categorized into 7 antigenic complexes: Barmah Forest (BF), Eastern Equine Encephalitis (EEE), Middelburg (MID), Ndumu (NDU), Semliki Forest (SF), Venezuelan Equine Encephalitis (VEE), and Western Encephalitis of Equine (WEE).6CHIKV is a spherical, enveloped, positive-stranded RNA virus (approximately 60–70 nm in diameter). The genome of the Chikungunya virus comprises 11,805 nucleotides and two polyproteins which possess one non-structural polyprotein of Capsid nsP1, nsP2, nsP3, and nsP4, as well as five-protein structural polyprotein composed of Capsid, E3, E2, 6 K, in addition to E1.5,7
The 5′ end of the RNA molecule is capped with 7-methylguanosine whereas the 3′ end is polyadenylated. An intermediate negative-stranded RNA is transcribed to form a positive-stranded sub genomic RNA which is called 26SRNA. Transcribed RNA is the mRNA for viral structural protein formation. Alphaviruses have retained domains that play a significant job in regulating the synthesis of viral RNA. All domains are discovered at both ends of the 5′ and 3′ and in the intergenic region. It is anticipated that the glycoprotein E1 and E2 will make heterodimers that match the surface uniformly as trimeric spikes present on the viral surface. The viral envelope made of glycoprotein plays a part in cell attachment.7
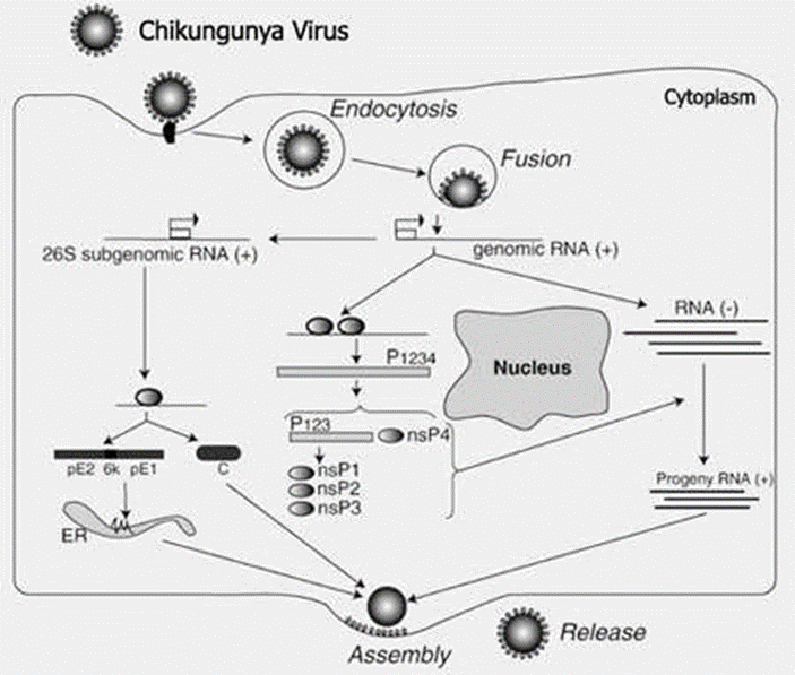
Virions situated on the surface of the cell membrane are uncoated and will enter into the host cells through the viral envelope’s fusion and endocytosis. The transcription site for mRNA is located in the cytoplasm of the cell. The process of replication is not restricted to a specific host, tissue, and organ, so the replication of the virus happens in different organs. The replication of the virus genome starts from creature host in the cytoplasm.8
EPIDEMIOLOGY
The first major instance of Chikungunya fever was recorded within the urban areas of Krung in the early 1960s.The irruptions of CHIKV were minor from time to time from 1st outbreak in 1963 up to 2014 and no major outbreaks were recorded till 2004.9 The reliable source of CHIKV was first observed and reported in Italy, and it was assumed that the transmission of that virus in India was the targeted source of CHIKV, the Homo sapiens, through viremia. The early instances of Chikungunya fever were unpredictable which induced a huge population, but in contrast, the instances of this virus vanished over a certain period of time.10 In India, the occurrence of CHIKV was first reported in Kolkata in 1963. In 1964 the instance of CHIKV was observed in the Indian southern east coastal regions of Chennai, Pondicherry, and Vellore. Nearly about 4,000,000 cases of Chikungunya were observed in the regions of Chennai which was about 65 -70 % of the total instances recorded that year. In 1965 instances of Chikungunya were recorded in the Visakhapatnam, Rajahmundry and Kakinada, Nagpur, and during 1973 new cases were recorded in Barsi, Tamil Nadu, and Maharashtra.11
No cases were reported after those recorded instances and it stated that CHIKV had vanished from India and South-East Asian regions, but a massive outbreak was reported in the current millennium as contrasted to the above declaration.10 After 32 years, the incidence of suspected CHIK instances was reported in India at the end of 2005 in coastal regions of Andhra Pradesh and Kerala, causing an explosion in 13 states. It has influenced various ages and genders. The incidents of CHIKV were confirmed in February and March 2006 in Andhra Pradesh, and this lead the World Health Organization to endorse the Chikungunya instances in India2. During the outbreak of 1963 to 1973, the isolated CHIKV belonged to the Asian genotype, but in contrast, the CHIKV isolated in the second instance during 2006 belonged to the African genotype.11 In 2008, nearly 100,000 people were infected by CHIKV in various villages in Kasargod, Kerala.3 This was followed by a major occurrence in the Thirunallar region of Tamil Nadu in 2009-2014. In the year 2009 and 2010, the cases of CHIKV belonging to the southeastern East African genotype (E1: 226A) was reported in Maharashtra, Goa, Rajasthan, West Bengal, Andaman and Nicobar islands, and Podokri.8,12 In 2011, the cases of CHIKV were observed in all states of India except, Dadar, Punjab, and Nagar Haveli, and Lakshadweep.13
TRANSMISSION
Transmission of CHIKV to humans occurs when the individual is hit by the infected Aedes-mosquitoes, especially the Aedes mosquitoes that generally stomp during the day. Recent outbreaks of CHIKV were reported as the vertical embryo transmission.14 Aedes. Albopictus also known as the Asian tiger mosquitoes is the most prevalent source for spreading the CHIKV in Asia.15
CHIKV has a 56- 60-hour window period from the moment of virus exposure. The patient’s viral load rises in the window period to various folds. The inhibition of hemagglutination and the antibody neutralization test after 5 days of exposure indicates a substantially positive result in viraemia.16
CLINICAL FEATURES
Chikungunya virus can infect both genders and all age groups. This disease has an incubation period starting from the 3rd day from the time of exposure up to 12 days, but symptoms mostly occur between 3-7 days.17 In immunocompromised people, the rate of instances will be higher for around 70 – 85% on contrasting the rate of instances with the normal exposed people.18 The symptoms initiate within 4 and 7 days of the patient being chomped by the CHIKV-infected vector. Symptoms include high fever (40°C/104°F), chills, cerebral pain, regurgitation, joint agony (lower back, lower leg, ankles, wrists, or phalanges), muscle torment, nausea, fatigue rash, and arthralgia. 17After the incubation period in a few first day’s patient may complain about headache, throat discomfort, abdominal pain, and constipation. Polyarthopathy by this infection regularly causes torment to the little joints and some bigger joints in the hands, wrists and lower legs, knees, and shoulders.19
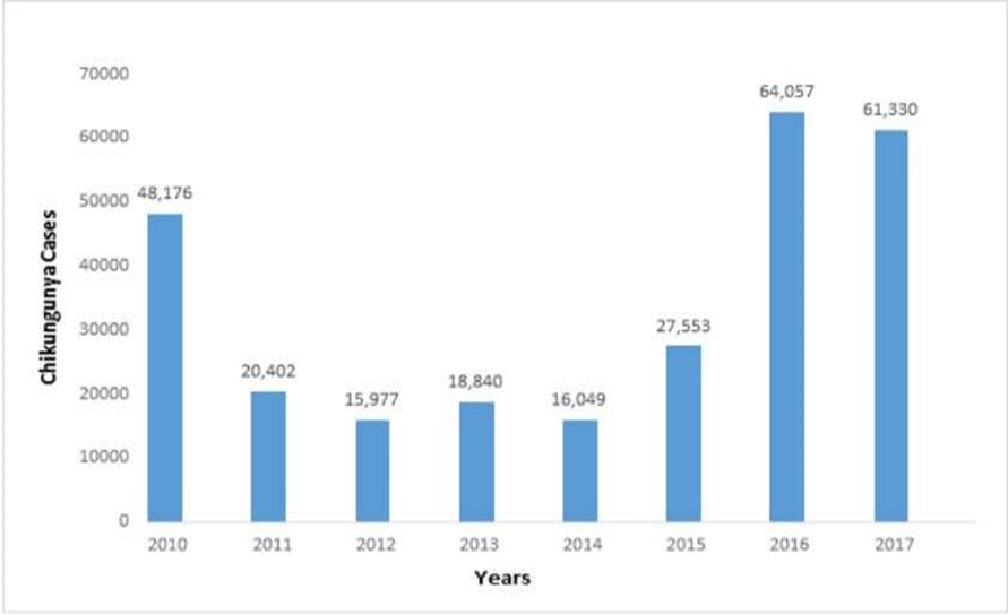
The symptoms are frequently hard to differentiate from dengue fever, as the Chikungunya fever exhibits the same pathogenicity of dengue fever, and furthermore, both dengue and CHIKV are transmitted by the same vector and most speculate patients are diagnosed for both dengue and CHIKV infections.20 Therefore, it is very necessary to differentiate the instances of CHIKV from that of Dengue through a proper channel of clinical diagnosis. Further, some neurological complications such as meningoencephalitis have been diagnosed among patients for the first time in the French Rhone islands and transmission of CHIKV from mother to fetus instances were also reported in these regions.21 The instances of Chikungunya can be confirmed by Four-fold HI antibody difference in opposite serum samples, Recognition of IgM antibodies, separation of CHIKV from serum, Isolation of nucleic acid of Chikungunya virus in sera by RT-PCR.22 According to the program that is National Vector Borne Disease Control Program (NVBDCP) nearly about 60000 instances of CHIKV were reported in India from 2010 to 2017.23
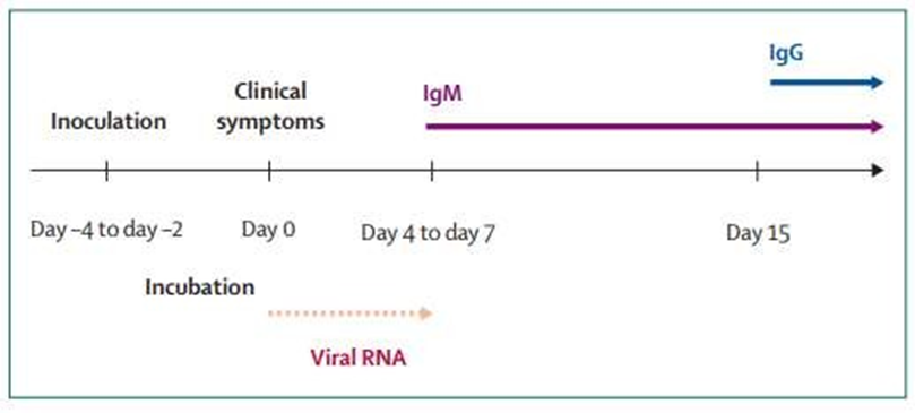
LABORATORY DIAGNOSIS
Chikungunya infections can identify by virus screening, isolation of viral RNA, or precise detection of antibodies in the patient samples. Typically, the sort of examination executed depends upon the timing and quantity of samples available.4 The most reliable test to identify Chikungunya is blood testing as the symptoms are like lethal dengue fever. For example, RT-PCR and serological tests are widespread laboratory tests for Chikungunya.24
RT–PCR
Viral RNA can be readily identified by RT-PCR in serum samples acquired from patients in the duration of the acute infection phase. Infection of Chikungunya induce elevated concentrations of viremia (up to 1x10E6.8 plaque-forming units per mL), usually enduring 4– 6 days after exposure to the virus.19 Therefore, RT-PCR can readily be performed within first 7 days on an acute-phase sample to identify infection with chikungunya virus. The products of RT–PCR from specimens can also be used for genotyping of viruses, facilitate comparisons with virus specimens from multiple ecological sources.20
ELISA
Anti-CHIKV immunoglobulin (Ig) M and IgG antibodies are detected from either acute or convalescent-phase samples by ELISA. Serological findings require further blood than other procedures.2 It is very precise with very minute cross-reactivity with associated alphaviruses. Samples testing from given instances discovered that Chikungunya-specific antibodies IgM grow quickly within days of infection and last for several months.11
IMMUNOFLUORESCENCE ASSAY
Immunofluorescence testing is delicate and definite, but it lacks the capacity to measure the number of antibodies which seems to be subjective and requires unique facilities and preparation.24
PRNT
PRNT is very useful because it is very specific to alphaviruses and is the gold standard for confirmation of serological test outcomes. The primary drawback to PRNT is that it is mandated to use live virus. Inspection at Biosafety level 3 (BSL-3) laboratories needing distinctive laboratory containment facilities.17
HEMAGGLUTINATION TEST
The acceptable technique of diagnosing the CHIKV instances is by using kinetic hemagglutination-inhibition tests to differentiate the Chikungunya strain. Chikungunya is affirmed when paired serum samples develop symptoms such as fever and joint pain together with a four-fold inhibition of hemagglutination antibody.1
Testing of samples in both acute and hospice phases, retrieved 3 weeks apart, should be adequate to verify the infection in patients suffering from elevated fever coupled with intense joint agony and latest trips to the region of Chikungunya instances were recorded. 1,24 Furthermore, Cryoglobulinemia has been revealed in several patients recently, consequently, if a person has an adequate clinical syndrome and travels to an impacted region, this should be regarded if the findings of serological tests are negative. For help with diagnostic testing, Health care professionals should contact their department of government or local health or the CDC.22,24
TREATMENT AND PREVENTION
Chikungunya fever treatment is symptomatic and effective. It can be useful to ensure appropriate fluid intake and proper use of paracetamol or NSAIDs. Aspirin should be prevented because of its effect on platelets.2 Electrolyte asymmetry, acute renal dysfunction pre-renal, bleeding manifestations should be closely monitored.10,11 Mosquito abatement is the appropriate strategy for combating an outbreak or even preventing future ones. There are no vaccines or antivirals for Chikungunya. Chloroquine is regarded as a feasible cure for Chikungunya-related diseases and as an antiviral agent in the fight against the Chikungunya virus.24,25
CONCLUSION
In India, several studies have been derived from the ongoing incidence of chikungunya. Chikungunya is a mosquito-borne viral disease that is not fatal. Chikungunya is a vector-borne disease and zoonotic disease. This study provides transparency to enhance understanding of various factors such as epidemiology, biological, dynamics, immunology, and the status of CHIKV infection in India. In addition, we grasped risk factors that trigger the growing incidence of Chikungunya and become aware of Chikungunya prevention awareness as well as present Chikungunya status in India.
REFERENCES
- World Health Organization. Regional Office for South-East Asia. Guidelines on clinical management of chikungunya fever. WHO Regional Office for South-East Asia. 2008.
- Chhabra M, Mittal V, Bhattacharya D, Rana U, Lal S. Chikungunya fever: a re-emerging viral infection. Indian J Med Microbiol. 2008 Jan-Mar;26(1):5-12. https://doi.org/10.4103/0255-0857.38850. PMID: 18227590.
- Kannan M, Rajendran R, Sunish IP, Balasubramaniam R, Arunachalam N, Paramsivan R, Tewari SC, Samuel PP, Tyagi BK. A study on chikungunya outbreak during 2007 in Kerala, south India. Indian J Med Res. 2009 Mar;129(3):311-5. PMID: 19491425.
- Economopoulou A, Dominguez M, Helynck B et al. Atypical Chikungunya virus infections: clinical manifestations, mortality and risk factors for severe disease during the 2005-2006 outbreak on Reunion. Epidemiol Infect. 2009; 137: 534–541.
- Santhosh SR, Dash PK, Parida MM, Khan M, Tiwari M, Rao PVL. Comparative full genome analysis revealed E1: A226V shift in 2007 Indian Chikungunya virus isolates. Virus Res. 2008; 135: 36–41.
- Arankalle VA, Shrivastava S, Cherian S et al. Genetic divergence of Chikungunya viruses in India (1963-2006) with special reference to the 2005-2006 explosive epidemic. J Gen Virol. 2007; 88:1967–1976.
- Abraham AM, Sridharan G. Chikungunya virus infection-a resurgent scourge. Indian J Med Res. 2007; 126: 502–505.
- Cherian SS, Walimbe AM, Jadhav SM et al. Evolutionary rates and timescale comparison of Chikungunya viruses inferred from the whole genome/E1 gene with special reference to the 2005-07 outbreak in the Indian subcontinent. Infect Genet Evol. 2009; 9: 16–23.
- Ravi V. Re-emergence of chikungunya virus in India. Indian J Med Microbiol. 2006 Apr;24(2):83-4. https://doi.org/10.4103/0255-0857.25175. PMID: 16687855.
- Cecilia D. Current status of dengue and chikungunya in India. WHO South East Asia J Public Health. 2014 Jan-Mar;3(1):22-26. https://doi.org/10.4103/2224-3151.206879. PMID: 28607250.
- Parashar D, Patil D. Chikungunya: a disease re-emerged in India after 32 years. A Rev Diam Jubil Publ. NIV Commem. Compend. Arankalle VA, Cecilia D NIV Golden to Diam Jubil Glorious Decad. 2012; 221–242.
- Lahariya C, Pradhan SK. Emergence of chikungunya virus in Indian subcontinent after 32 years: A review. J Vector Borne Dis. 2006 Dec;43(4):151-60. PMID: 17175699.
- Soumahoro MK, Gérardin P, Boëlle PY, Perrau J, Fianu A, Pouchot J, Malvy D, Flahault A, Favier F, Hanslik T. Impact of Chikungunya virus infection on health status and quality of life: a retrospective cohort study. PLoS One. 2009 Nov 11;4(11):e7800. https://doi.org/10.1371/journal.pone.0007800. PMID: 19911058; PMCID: PMC2771894.
- Nigam A, Sharma S, Jain A, Gupta A, Prakash A. Vertical Transmission of Chikungunya Manifesting as Foetal Pericardial Effusion. J Assoc Physicians India. 2016 Dec;64(12):76-79. PMID: 28405994.
- Inamadar AC, Palit A, Sampagavi VV, Raghunath S, Deshmukh NS. Cutaneous manifestations of chikungunya fever: observations made during a recent outbreak in south India. Int J Dermatol. 2008 Feb;47(2):154-9. https://doi.org/10.1111/j.1365-4632.2008.03478.x. PMID: 18211486.
- Ramful D, Carbonnier M, Pasquet M, Bouhmani B, Ghazouani J, Noormahomed T, Beullier G, Attali T, Samperiz S, Fourmaintraux A, Alessandri JL. Mother-to-child transmission of Chikungunya virus infection. Pediatr Infect Dis J. 2007 Sep;26(9):811-5. https://doi.org/10.1097/INF.0b013e3180616d4f. PMID: 17721376.
- Edelman R, Tacket CO, Wasserman SS, Bodison SA, Perry JG, Mangiafico JA. Phase II safety and immunogenicity study of live chikungunya virus vaccine TSI-GSD-218. Am J Trop Med Hyg. 2000 Jun;62(6):681-5. https://doi.org/ 10.4269/ajtmh.2000.62.681. PMID: 11304054.
- Brighton SW, Simson IW. A destructive arthropathy following Chikungunya virus arthritis–a possible association. Clin Rheumatol. 1984 Jun;3(2):253-8. https://doi.org/10.1007/BF02030766. PMID: 6088159.
- Martins HA, Bernardino SN, Santos CC, Ribas VR. Chikungunya and Myositis: A Case Report in Brazil. J Clin Diagn Res. 2016 Dec;10(12):OD05-OD06. https://doi.org/10.7860/JCDR/2016/23680.9053. Epub 2016 Dec 1. PMID: 28208912; PMCID: PMC5296485.
- Mohan A. Chikungunya fever: clinical manifestations & management. Indian J Med Res. 2006 Nov;124(5):471-4. PMID: 17213512.
- Cunha RVD, Trinta KS. Chikungunya virus: clinical aspects and treatment – A Review. Mem Inst Oswaldo Cruz. 2017 Aug;112(8):523-531. https://doi.org/10.1590/0074-02760170044. PMID: 28767976; PMCID: PMC5530543.
- Krishnamoorthy K, Harichandrakumar KT, Krishna Kumari A, Das LK. Burden of chikungunya in India: estimates of disability adjusted life years (DALY) lost in 2006 epidemic. J Vector Borne Dis. 2009 Mar;46(1):26-35. PMID: 19326705.
- Kumar PSS, Arjun MC, Gupta SK, Nongkynrih B. Malaria, dengue and chikungunya in India-An update. Indian J Med Spec. 2018; 9: 25–29.
- Organization WH and others, Guidelines for prevention and control of chikungunya fever. 2009.
- Organization WH, for Research SP, in Tropical Diseases T, of Control of Neglected Tropical Diseases, WHOD, Epidemic WHO, Alert P. Dengue: guidelines for diagnosis, treatment, prevention and control World Health Organization, 2009.
ARTICLE TYPE: Review Article;
ORCID ID; Open Researcher and Contributor Identifier (ORCID) ID of corresponding author: https://orcid.org/0000-0002-5264-6546;
ETHICAL: NA; ACKNOWLEDGEMENT: None;
FINANCIAL DISCLOSURE: The authors declare that there was no financial aid received.;
CONFLICT OF INTEREST: No conflict of interest associated with this research work.;
AUTHORS CONTRIBUTION: All authors participated equally.;
CORRESPONDING AUTHOR AFFILIATIONS: Dr. Louis Cojandaraj, Assistant Professor, Department of Medical Laboratory Sciences, Lovely Professional University, Phagwara, Punjab 144411, India.;
CORRESPONDING AUTHOR EMAIL: louis.23330@lpu.co.in;
ARTICLE CITATION: Bahaduri MA, Cojandaraj L, Kataria J. Epidemiology of chikungunya in India—a short review. SALT J Sci Res Healthc. 2022 March 04; 2(1): 40-45.
PUBLISHER’S NOTE: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.




© Mohammad Aman Bahaduri, Louis Cojandaraj, Juhi Kataria.
Originally published in the SALT Journal of Scientific Research in Healthcare (https://saltjsrh.in/), 04.03.2022.
This is an open-access article distributed under the terms of the Creative Commons License (https://creativecommons.org/licenses/by-nc-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the SALT Journal of Scientific Research in Healthcare ((https://saltjsrh.in/), is properly cited. The complete bibliographic information, a link to the original publication on https://saltjsrh.in/, as well as this copyright and license information must be included.
