STUDY ON EFFICACY OF COMMERCIALLY AVAILABLE MOUTH WASH
SALT Journal of Scientific Research in Healthcare
Volume 1, Issue 2, Page 24-34, Published on 29th October 2021
https://doi.org/10.56735/saltjsrh.ms2101022434
Research Article
LEYON SELVIN RAJ A, JIGNASA H BERA, KUSHANI GIRISHBHAI BHAINE AND DHARA N PATEL
*Corresponding Author: Dr Dhara N Patel, Assistant Professor, Department of Medical Laboratory Science, Bapubhai Desaibhai Patel Institute of Paramedical Science (BDPIPS), Charotar University of Science and Technology, Charusat Campus, Changa 388421, Dist. Anand , Gujarat, India.
ORCID ID: 0000-0002-4330-8158
Email: dharapatel.cips@charusat.ac.in
ABSTRACT
Mouthwash is an aqueous solution with antibacterial, deodorant, and refreshing properties. Four different kinds of commercial mouthwashes were compared in this study to test their efficacy using the disc diffusion method; a total of forty-three oral swabs were collected. Compared to the inhibition zones of four mouthwash brands, the chlorhexidine gluconate-containing mouthwash had a better antibacterial effect.
Keywords: Mouth wash, disc diffusion method, antimicrobial, antiseptic
1.0 INTRODUCTION
The need to prevent human disease is well recognised and is related to making the occurrence or progression of a disease process unlikely or impossible. Because the mouth is the doorway to the human body, oral health is crucial. Bacteria are always present in the oral cavity, and if the dental plaque biofilm is not eliminated on a regular basis, it can contribute to the development of oral illness. The merits of daily oral hygiene to oral health have long been understood (Axelsson et al., 2004)
The oral microbiome is a complex ecological system where up to 700 species of microorganisms have been identified (Palmer et al., 2008). Some of the predominant groups present in the mouth include Streptococcus, Neisseria, Veillonella, Actinomyces and other obligate anaerobes (Avila et al., 2009). These organisms maintain a mutualistic relationship with the host by preventing pathogenic species from adhering to the mucosal surface (Liljemark and Bloomquist 1996). Oral micro florae can cause dental plaques and are also a common cause of dental caries and periodontal disease (Kigure et al., 1995). Oral microflora are most commonly found in gingival crevices, coronal plaques, tongue dorsum, buccal mucosa and saliva (Gibbons and Houte, 2000).
Bacteria thrive in the ecological niche created by the tooth surface as well as the gingival epithelium. However, a highly efficient innate host defence system constantly monitors bacterial colonisation and prevents bacterial invasion of local tissues. A dynamic equilibrium exists between dental plaque bacteria and the innate host defence system (Rogers, 2008). Oral bacteria include Streptococci, Lactobacilli, Staphylococci, Corynebacteria, and various anaerobes in particular Bacteroides. The oral cavity of the newborn baby does not contain bacteria but rapidly becomes colonised with bacteria such as Streptococcus salivarius. With the appearance of the teeth during the first year, colonisation by Streptococcus mutans and Streptococcus sanguinis occurs as these organisms colonise the dental surface and gingiva. Other streptococci strains cling to the gums and cheeks but not to the teeth. A variety of anaerobic species can be found in the gingival crevice area (the supporting elements of the teeth) (Rogers, 2008).
1.1 Streptococcus mutans
Streptococcus mutans is a facultatively anaerobic, Grampositive coccus shaped bacterium commonly found in the human oral cavity and is a significant contributor to tooth decay (Ryan and Ray, 2004 & Loesche, 1924). They cause tooth decay by metabolising sucrose into lactic acid with the help of an enzyme called glucansucrase. The acidic environment created in the mouth by this process causes the highly mineralised tooth enamel to be vulnerable to decay. S. mutans is one of a few specialised organisms equipped with receptors that improve adhesion to the surface of teeth. S. mutans uses sucrose to make a sticky extracellular dextran-based polysaccharide that permits them to cohere and form plaque. The enzyme dextransucrase (a hexosyltransferase) in S. mutans makes dextran utilising sucrose as a substrate in the following reaction:
n sucrose → (glucose)n + n fructose
Sucrose is the only sugar that bacteria can use to form this sticky polysaccharide (Ryan and Ray, 2004). Many other carbohydrates, such as glucose, fructose, and lactose, can be digested by S. mutans, but the final product is lactic acid. The combination of plaque and acid leads to dental decay (Madigan and Martinko 2005). Streptococci represent 20% of the oral bacteria and actually determine the development of the biofilms. Although S. mutans can be antagonised by pioneer colonisers, once they become dominant in oral biofilms, dental caries can develop and thrive (Biswas and Biswas, 2011).
1.2 Lactobacillus species
Some Lactobacillus species have been linked to dental caries instances. Lactic acid corrodes teeth, and for many years, the Lactobacillus count in saliva has been employed as a “caries test.” One of the arguments in favour of the usage of fluoride in toothpaste is this. Lactobacilli characteristically cause existing carious lesions to progress, especially those in coronal caries (Twetman and StecksénBlicks, 2008 & Meurman and Stamatova, 2007).
1.3 History of Mouthwash
The principle that plaque biofilm is the major etiologic factor causing gingivitis provides the justification for the use of antimicrobial mouth rinses (Loe et al., 1965). Mouth rinse has been a part of human culture for more than 2000 years. The first mouthwash advocated for dental plaque reduction seems to be urine from a child or, even better, from a newborn baby (Weinberger, 1948). Willoughby D. Miller (a dentist with a background in microbiology) was the first to recommend using an antibacterial mouthwash containing phenolic compounds to treat gingival inflammation in the 1880s. (Jackson, 1997). Mouthwashes have been commonplace in recent decades, usually after mechanical plaque biofilm management. Mouthwashes are an excellent vehicle for incorporating chemicals and are popular among the general population due to their ease of use, plaque biofilm reduction, and breath-freshening impact.(Moran 1997, Cummins & Creeth 1992 and Cummins 1997). With fierce competition among independent manufacturers for a piece of this market, a variety of efficacy claims have been made, employing a variety of terminologies to indicate efficacy. Although people in industrialised countries use various oral hygiene products with the expectation of an oral health benefit, it is important that sufficient scientific evidence exists to support such claims. Dental professionals have choices and make decisions every day as they advise their patients (Suvan and D’Aiuto 2008).
1.4 Mouthwash (Mouthrinses)
A mouthwash is a non-sterile aqueous solution that is primarily used for its deodorant, refreshing, or antibacterial properties. Mouthwashes or rinses are designed to reduce oral bacteria, remove food particles, temporarily reduce bad breathe and provide a pleasant taste. Chlorhexidine, essential oils, triclosan, cetylpyridinium chloride, sanquinarin, sodium dodecyl sulphate, and other metal ions have all been tested for their plaque-reducing efficiency and capacity to reduce mutans streptococci in mouth rinses (tin, zinc, copper).
The FDA classifies mouthwashes as either aesthetic or medicinal or a combination of the two.
The cosmetic mouth rinses are over-the-counter products that are mainly intended as mouth fresheners. Therapeutic rinses are available on prescription or over-the-counter products with added active ingredients and are marketed as anti-plaque/antigingivitis and anticaries drug products.
Cosmetic rinses are over-the-counter commercial solutions that assist eliminate oral debris before or after brushing, suppress lousy breath temporarily, reduce bacteria in the mouth, and refresh the mouth with a pleasant taste.
Therapeutic rinses have many of the same benefits as aesthetic rinses, but they also contain an additional active ingredient, such as fluoride or chlorhexidine, which can help guard against some dental illnesses.
The amount of various ingredients in mouthwashes varies from one product to the next. Some practically have the same composition as toothpaste, although they do not contain abrasives. Distinct from toothpaste, most mouth rinses contain alcohol as a preservative and a semiactive ingredient. The amount of alcohol is usually ranging from 18 to 26 per cent.
Mouthrinse formulas are typically simpler than dentifrice formulations, and compatibility issues aren’t as prevalent as they are with dentifrices. The oldest and simplest used mouth rinse has been a dilute saline solution.
1.5 Ingredients
Humectant: Sorbitol and glycerin to prevent drying.
Surfactant: Helps to keep ingredients in solution.
Alcohol: To enhance antibacterial activity and taste. Also, to help keep flavouring agents in solution.
Antibacterial agents: Quaternary ammonium compounds such as cetylpyridinium chloride, benzethonium chloride, and povidineiodine, sodium lauryl sulphate, zinc citrate trihydrate, triclosan, and metal salts are the most often used antibacterial agents.
Sweetening agents: Saccharin.
Flavouring agents: Spearmint, peppermint, eucalyptus and menthol are often used as flavouring agents in mouthwashes. The flavouring agents are solubilised and dispersed through liquid via the detergent.
Therapeutic Rinses: Fluoride containing: Sodium fluoride (NaF) mouthrinse has been used as 0.2 per cent for weekly rinse and 0.05 per cent for daily rinsing. Because of its inexpensive cost, ease of use, and pleasant taste, it is the most extensively used fluoride rinse.
Chlorhexidine Rinses: Chlorhexidine digluconate, useful in decreasing gingivitis and plaque buildup, is an active ingredient in certain ADA-approved commercial mouth rinses. It is one of two mouth rinses shown to reduce gingivitis in long-term clinical trials and appears to be the most effective anti-plaque and antigingivitis agent known today.
However, since the effect of chlorhexidine is influenced by anionic tensides such as sodium lauryl sulphate, when brushing with a toothpaste that contains sodium lauryl sulphate, wait at least 30 minutes before rinsing with a CHX mouthrinse.
CHX 0.2 per cent is suitable as a supportive measure during treatment of gingivitis and periodontitis, but it should not be used for longer than two weeks. After this, however, it is important to restore healthy oral flora (Marya, 2011).
2.0 REVIEW OF LITERATURE
Patters et al., (1986) studied on effects of octenidine mouth rinse on plaque formation and gingivitis in humans. Their study suggested that octenidine mouth rinse is well tolerated and extremely effective in inhibiting plaque accumulation and gingivitis when used for 21 days without mechanical oral hygiene. Further evaluation of the usefulness of octenidine as a therapeutic modality will require studies of greater than 21 days and determination of the ability of octenidine to reverse existing gingivitis.
Haim Tal and Mel Rosenberg (1990) A simple, non-invasive test (the Oral test) has recently been proposed, which provides an estimate of oral microbial levels based on the rate of oxygen depletion in expectorated milk samples. In the study, Oratest scores were compared to clinical parameters (Plaque Index [PI] and Gingival Index [GI]. The findings show that the Oratest can accurately predict gingival inflammation, extending previously documented robust links between Oratest scores and bacteria counts. According to the findings, the Oratest could be useful as a therapeutic and research tool.
Furuichi et al., (1996) studied on effects of mouth rinses containing salifluor on de novo plaque formation and developing gingivitis. Their clinical trials demonstrated the potential of salifluor as an effective anti-plaque and anti-inflammatory agent.
Luca Francetti et al., (2000) Chlorhexidine spray versus chlorhexidine mouthwash in the control of dental plaque after periodontal surgery. The current findings suggest that the efficacy of CHX spray in controlling dental plaque after surgery is comparable to that of CHX mouthwash. In the CHX spray group, however, tooth discoloration was dramatically reduced. The observed effects might be related to the way of delivering CHX and to the total dose administered, about 80% lower in group B in respect to A. More research is needed to confirm the preliminary findings of this study.
Philip D. Marsh (2000) Studied the role of microflora in health and demonstrated that the resident oral microflora plays an active role in the normal development of the mouth and the maintenance of health at a site. Clinicians must be aware of the positive characteristics of resident microflora, and treatment techniques should focus on controlling rather than eliminating these organisms, particularly in dental plaque.
Mat Ludin and Md Radzi (2001) studied seven different brands of mouthwashes that were assessed to inhibit the growth of oral microorganisms. The efficiency of the formulations including cationic surfactants and complex organic nitrogenous chemicals was higher than that of the older formulations based on phenols. The mouthwashes were ranked according to their antibacterial activity, which did not always match the manufacturer’s claims or usage instructions.
Valeria Marinho et al., (2003) stated that fluoride is a mineral that prevents tooth decay (dental caries). Since the widespread use of fluoride toothpaste and water fluoridation, the value of additional fluoride has been questioned. Fluoride mouth rinse is a concentrated solution that needs to be used regularly to have an effect. The review of trials found that regular use of fluoride mouth rinse reduces tooth decay in children, regardless of other fluoride sources. There would be less decay in one out of every two children with high levels of tooth decay (and one out of every 16 with the lowest levels). However, more research is needed on the adverse effects and acceptability of mouth rinses.
Khalid Almas et al., (2005) Miswak extract was compared to commercially available non-alcohol mouth rinses in an in vitro antibacterial study. Mouthrinses containing chlorhexidine was with maximum antibacterial activity, while cetylpyridinium chloride mouth rinses were with moderate and miswak extract was with low antibacterial activity.
Paul Bahna et al., (2006) developed an efficacious and non-irritant mouthwash that is alcohol-free and has a low concentration of chlorhexidine to be used for preventing oral cavity infections immunocompromised and cancer patients. The developed alcohol-free mouthwash solution, with a low chlorhexidine concentration, showed antiseptic effect against planktonic and biofilm forms of C. albicans and against K. pneumoniae, P. aeruginosa, and MRSA.
John C. Gunsolley (2006) In six-month studies, the author conducted a comprehensive assessment of the literature to assess the efficacy of antigingivitis and antiplaque products. He searched electronic databases for six-month randomised clinical studies that evaluated both anti-plaque and antigingivitis properties of dentifrices or mouth rinses. In addition, the author asked manufacturers for unpublished studies. The findings in this systematic review show that several agents have anti-plaque and anti-gingivitis properties. These findings back up the usage of these products as part of a standard oral hygiene routine.
Andrew Rawlinson et al., (2008) studied the efficacy of two alcohol-free cetylpyridinium chloride mouthwashes – by a randomised, double-blind crossover study. The study concluded that the use of both CPC mouthwashes resulted in less plaque accumulation compared with the control. And there were no statistically significant differences in plaque accumulation between the two CPC mouthwashes were found.
Evandro Watanabe et al., (2008) Studied on the maximum inhibitory dilution (MID) of four Cetylpyridinium Chloride (CPC) – Based mouthwashes: CPC + propolis, CPC + malva, CPC + eucalyptol + juá + romã + propolis (natural honey®) and CPC (cepacol®). The data was subjected to the Kruskal-Wallis test, which revealed that the mid of cepacol® was lower than that of the other products (p<0.05). In conclusion, CPC-mouthwashes showed antimicrobial activity against S. aureus, and the addition of other substances to CPC improved its antimicrobial effect.
Kamal Rai Aneja et al., (2010) studied the antimicrobial potential of ten often used mouthwashes against four dental caries pathogens. Hexidine mouthwash (ICPA Health Products Ltd., Ankleshwar, India) showed excellent antimicrobial activity against the four dental caries causing microorganisms in vitro. The six mouthwashes are found to be effective against all the four tested microorganisms at all the four concentrations, comprising of Chlorhexidine gluconate as the basic constituent, presented different antimicrobial activities.
Nattapon Sritrairat et al., (2010) aimed a study to determine the antifungal activity of lawsone methyl ether mouthwash (LME) in comparison with chlorhexidine mouthwash (CHX) in vitro and in vivo. In which lawsone methyl ether mouthwash possesses potent antifungal activity both in vitro and in vivo. And they stated that the concentration of the mouthwash needs to be adjusted in addition to further clinical trials on long-term use.
Mahin Bakhshi et al., (2011) compared the therapeutic effect of aqueous extract of garlic and nystatin mouthwash in denture stomatitis. And they concluded that the efficacy of garlic and lack of side effects for this compound and also regarding the nystatin-associated complications, garlic extract can be introduced as a substitution for standard treatment in DS.
Dalirsani et al., (2011) compared the antimicrobial activity of ten herbal (thyme, mint, garlic, cinnamon, chamomile, tea tree, clove, spearmint, sage, and rosemary) extracts against Streptococcus mutans with chlorohexidine. Rosemary was found as a potent antimicrobial plant, and they suggested more studies for the production of herbal mouthwashes.
Fernanda Lessa et al., (2012) worked on the efficacy of guaco mouthwashes (Mikania glomerata and Mikania laevigata) on the disinfection of toothbrushes. Moreover, they concluded that treatment with chlorhexidine and M. glomerata were statistically similar. And M. glomerata mouthwash could be useful in herbal strategy programs against Streptococci mutans.
Wipawee Nittayananta et al., (2013) studied on effects of lawsone methyl ether mouthwash on oral Candida in HIV-infected subjects and subjects with denture stomatitis and concluded that the use of LME mouthwash for two weeks neither led to antifungal drug resistance nor significant changes in genotype of oral Candida. As a result, LME could be an alternate mouthwash for those who are at risk of acquiring oral candidiasis.
Majed M. Masadeh et al., (2013) studied the antimicrobial activity of common mouthwash solutions on multi-drug resistance bacterial biofilms. Their results showed that common mouthwash solutions have variable antibacterial activity depending on their major active components. Only mouthwash solutions containing chlorohexidine gluconate or cetylpyridinum chloride were effective against the majority, but not all, of the biofilm-forming bacteria, tested. In addition, bacteria in biofilms are less vulnerable to all mouthwash solutions than bacteria in planktonic stages.
Naiana Da Silva et al., (2013) studied on antimicrobial activity of mouthwashes and herbal products against dental biofilm- forming bacteria. In which the bacterial species were resistant to the tinctures and Listerine, but were susceptible to chlorhexidine, Malvatricin and periogard, and the test herbs did not show any inhibitory action against the tested biofilm-forming bacteria.
Karen Smith et al., (2013) evaluated and compared the activity of oral mouthwashes against biofilm forms of MRSA isolated from the oral cavity and the bloodstream. The time-kill kinetics efficacy of 7 over-the-counter mouthwashes was tested against 28 clinical MRSA biofilm isolates for 0.5, 1 and 2 min. In that, treatments of MRSA biofilms formed by oral and bloodstream isolates were not significantly different, with mouthwashes displaying a rapid and modest anti-biofilm effect. None of the biofilm isolates was completely eradicated by the compounds tested, with a maximal killing of only approximately 70% shown by Corsodyl and Peroxyl. After 0.5 minutes, all of the substances tested had reached their peak activity. Fluorigard showed the poorest overall activity (57% reduction).
R. Shahakbari et al., (2014) The objective of this study was to evaluate the effectiveness of green tea mouthwash in controlling the pain and trismus associated with acute pericoronitis in comparison to chlorhexidine (CHX) mouthwash. Conclusion and concluded that green tea mouth rinse could be an appropriate and effective choice for controlling pain and trismus in acute pericoronitis.
Quintas V et al., (2015) To determine the influence of Chlorhexidine substantivity on the re-growing period of salivary bacteria, researchers measured the substantivity of a single 0.2 percent Chlorhexidine mouthwash in saliva after it was neutralised with toothbrushing and 1 percent acetic acid. In the study, the immediate antibacterial effect of a single 0.2% Chlorhexidine mouthwash is so potent that the bacterial population needs more than three h to return to baseline bacterial vitality levels. The substantivity of a 0.2% Chlorhexidine mouthwash is a property that significantly increases its antibacterial activity from the first hour and contributes to extending the duration of its effect by at least double.
Fridus A. Van der Weijden, et al., (2015) Summarised and appraised the current state of evidence-based systematic reviews with respect to the efficacy of various active ingredients of over-the-counter chemotherapeutic mouthwash formulations for plaque control in managing gingivitis. According to the evidence, a mouthwash containing CHX is the best option. EO is the most reliable option for plaque control. In terms of gingivitis, there was no difference between CHX and EO.
Francisco A. V. Santos et al., (2015) The essential oil of Plectranthus amboinicus, a plant utilised by the local population for the treatment of oral ailments, was tested for chemical composition and antibacterial activity against a strain of Streptococcus mutans, either alone or as a mouthwash. Although the essential oil in combination with mouthwash was efficient in suppressing bacterial growth, it was less effective than chlorhexidine alone. This indicated the necessity of more studies about these combinations.
Nesma Sultan Mohamed et al., (2015) aimed to evaluate the effects of three different mouthwashes on the incidence of cyclosporine-A-induced gingival overgrowth. In the study, essential oils and chlorhexidine gluconate mouthwashes significantly reduced the incidence of gingival overgrowth compared with cetylpyridinium chloride.
Karpiński and Szkaradkiewicz (2015) studied pharmaco-biological activity and application of chlorhexidine and concluded that CHX (chlorhexidine) plays a valuable role in dentistry and antisepsis. It can also cause side effects.
3.0AIM AND OBJECTIVES
AIM: To study the effectiveness of four commonly available mouthwashes against normal oral microflora.
OBJECTIVE
To study the effectiveness of mouthwashes in controlling the microbial load in the mouth.
To compare their efficacy with different brands of mouthwashes in different subjects.
4.0 MATERIALS AND METHODS
4.1 COLLECTION OF MOUTHWASHES
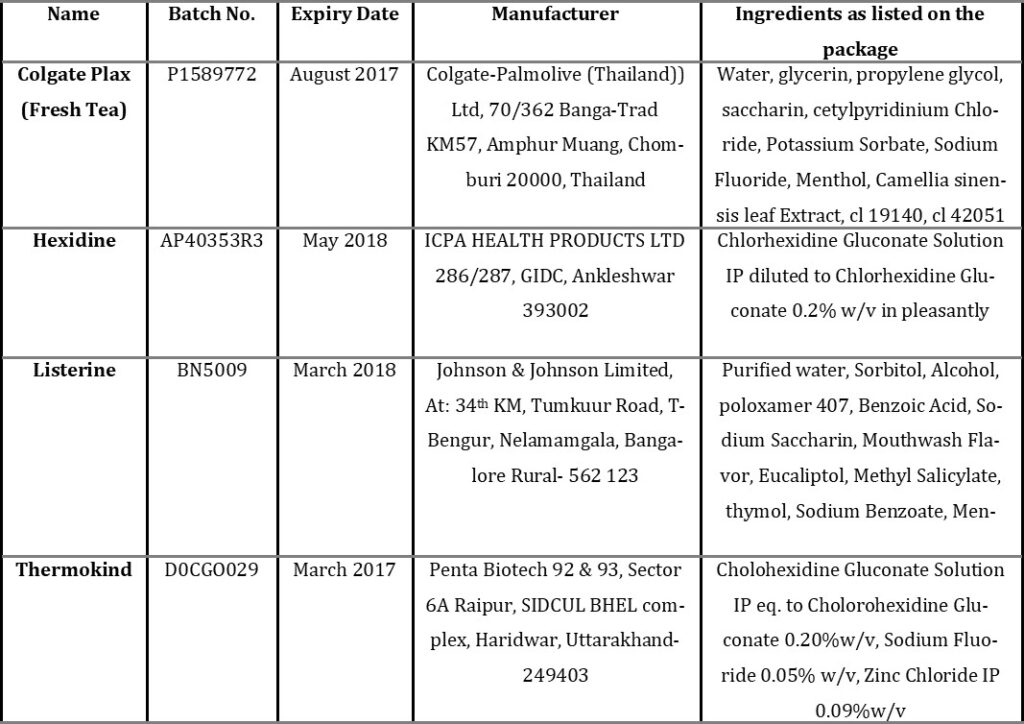
Four commercial mouth wash products were purchased from a drug store in Choolaimedu, Nungambakkam, Chennai.
4.2 SAMPLE COLLECTION
Sterile Swab (Hi media): Swab is collected from the buccal mucosa of healthy subjects by using a sterile swab. The subject is asked to wide open their mouth, and the swab from both left and right sides is collected carefully.
4.3 ANTIBACTERIAL ACTIVITY
The antibacterial activity of the mouthwash was tested by using the Kirby-Bauer Disk Diffusion test. The most popular method for determining antibiotic resistance/susceptibility is the Kirby-Bauer (K-B) disc diffusion test. These tests aid clinicians in determining which antibiotics to employ while treating a sick patient.
The Kirby-Bauer Disk Diffusion Test: The Kirby-Bauer (K-B) test utilises small filter disks impregnated with a known concentration of antibiotics. The discs are inoculated with the test microorganism and placed on a Mueller-Hinton agar plate. Antibiotic diffuses from the disc into the surrounding agar during incubation. If the test organism is susceptible to the antibiotic, it will be unable to develop in the immediate vicinity of the disc, resulting in a zone of inhibition.
Materials Used: Muller Hinton Agar Medium, Distilled Water, Conical Flask, Petri Plates, Laminar Airflow Chamber, Test Mouthwashes and Sterile Antibiotic Discs.
Muller Hinton Agar Medium
Formula / Liter
| Beef Extract | 2 g |
| Acid Hydrolysate of Casein | 17.5 g |
| Starch | 1.5 g |
| Agar | 17g |
| Final pH | 7.3 ± 0.1 |
| at 25°C |
Media Preparation: Suspend 38 g of the medium in one litre of purified water. Heat for one minute with frequent agitation to completely dissolve the medium. Autoclave at 121°C for 15 minutes. Cool to room temperature. Pour cooled Mueller Hinton Agar into sterile Petri dishes on a level, horizontal surface to obtain consistent depth. Allow cooling to room temperature. C.° 0.1 at 25±5. Check the pH of the Mueller Hinton Agar to make sure it’s at least 7.3.
Procedure: The antibacterial activity of the selected mouth wash was performed in the Muller Hinton Agar medium. 10ml of Muller Hinton Agar was poured in a sterile Petri plate, and it was allowed to solidify. The buccal swab is seeded on Muller Hinton Agar. After solidification, the filter paper disc (5mm in diameter) with mouth wash was placed on seeded plates. The antimicrobial assay plates were incubated at 370C. The diameter of the inhibition zones was measured in mm after 48 hours.
5.0 RESULT
Effectiveness of Mouthwash in Controlling the Oral Micro Flora: Four brands of mouthwashes (Colgate Plax, Hexidine, Listerine, Thermokind) were studied for their effectiveness in controlling the microbial load in the mouth, and it was found that Hexidine, Thermokind are very effective in controlling microbial load than the other two brands; and Colgate Plax showed a significant effect, and Listerine showed no significant effect.
Comparison of the Efficacy of the Mouth Washes in Different Subjects: The efficacies of the mouthwashes were compared by the effects on various subjects. Oral swabs were taken from 43 subjects, and the activities of the mouthwashes were measured by their antimicrobial activity on the oral microbes obtained by the oral swab. Comparatively, Hexidine and Thermokind showed a maximum efficacy with an average zone of inhibition of 13.1mm and 11.1. Colgate Plax showed a minimum efficacy with an average zone of inhibition of 9.4mm Listerine shows no significant effects on the oral samples obtained from the subjects.
6.0 DISCUSSION
Following the completion of the techniques to assess the antimicrobial potential of the mouthwashes, Of the four mouthwashes tested, Hexidine and Thermokind mouthwash emerged as the most effective antimicrobial mouthwash, based on the formation of a zone of inhibition produced by the mouthwash against the oral microbes collected from the 43 subjects, followed by Colgate Plax showed a significant level of activity, while Listerine lacked antimicrobial activity.
Interestingly, all three mouthwashes that showed excellent antimicrobial activities had Chlorhexidine gluconate as the active ingredient. Chlorhexidine gluconate is a cationic biguanide with broad-spectrum antimicrobial action, whose effectiveness in decreasing the formation of dental biofilm (plaque) and gingivitis has been demonstrated in several clinical studies (Aneja et al., 2010). Its mechanism of action is that the cationic molecule binds to the negatively-charged cell walls of the microbes, destabilising their osmotic balance (Amornchat et al., 2006 Hugoson et al., 1990)
Its substantivity, or ability to be kept in a specific environment, is related to its ability to attach to the carboxyl groups of the mucin that covers the oral mucus and be continuously released in an active state from these locations, displaced by calcium ions separated by the salivary glands (Silla et al., 2008).
From the overall results obtained, it is evident that the Hexidine and Thermokind mouthwashes listing Chlorhexidine gluconate as the active ingredient presented different antimicrobial activities. This is most likely owing to the various formulas of mouthwashes, as well as other substances. The possible explanation may be that the active product concentration and its interaction with other constituents and differences in the formulations might be responsible for different effects. The result justifies the antimicrobial claims of the mouthwashes made by earlier workers (Mat Ludin and Md Radzi 2001, Barnett 2006 & Pourabbas et al., 2005).
7.0 SUMMARY AND CONCLUSION
A mouthwash is a non-sterile aqueous solution that is primarily used for its deodorant, refreshing, or antibacterial properties. Mouthwashes or rinses are designed to kill bacteria in the mouth, remove food particles, alleviate bad breath temporarily, and have a nice flavour. Mouth rinses are usually divided into two categories: aesthetic and therapeutic, or a combination of the two. Cosmetic rinses are over-the-counter commercial solutions that assist eliminate oral debris before or after brushing, suppress bad breath temporarily, reduce bacteria in the mouth, and refresh the mouth with a pleasant taste. Therapeutic rinses often have the benefits of their cosmetic counterparts but also contain an added active ingredient, ex. fluoride or chlorhexidine that helps protect against some oral diseases. The amount of various ingredients in mouthwashes varies from one product to the next. Some practically have the same composition as toothpaste, although they do not contain abrasives. Distinct from toothpaste, most mouth rinses contain alcohol as a preservative and a semi-active ingredient. The amount of alcohol usually ranges from 18– 26% (Silje Storehagen and Shilpi Midha, 2003). The present study involves the study and comparison of the effectiveness of four different brands of commercial mouthwashes.
The mouth wash brands were collected from a local drug store and used for the study. The oral swabs were used as a microbial sample. Totally 43 oral swabs were collected from different subjects, and the mouthwashes were tested for their efficacy by the disc diffusion method. The efficacies of the different brands of mouthwashes were compared by their zone of inhibition. In that, it is found that the Hexidine and Thermokind show great efficacy, and their basic composition includes Chlorohexidine any way, they have shown a bit different in their efficacy in the zone of inhibition that is maybe because of their other composition. The Colgate Plax mouthwash showed a significant amount of antimicrobial effect. And Listerine showed no antimicrobial effect on any samples, and it is concluded that the composition of Listerine is ineffective.
Hexidine and Thermokind showed excellent antimicrobial activity against the oral microbes collected from the 43 subjects comparatively. Colgate Plax mouthwashes were found to be significantly effective against the oral microorganisms, and Listerine showed no effects against the microbial test sample, the composition of Listerine mouthwash is ineffective against the test microbes. The mouthwash containing Chlorhexidine gluconate showed a better antimicrobial effect, proving chlorohexidine a better antiseptic that the differences in the formulations would be responsible for different effects.
REFERENCES
- Almas K, Skaug N, Ahmad I. An in vitro antimicrobial comparison of miswak extract with commercially available non-alcohol mouthrinses. Int J Dent Hyg. 2005 Feb;3(1):18-24. https://doi.org/10.1111/j.1601-5037.2004.00111.x. PMID: 16451373.
- Amornchat C, Kraivaphan P, Dhanabhumi C, Tandhachoon K, Trirattana T, Choonhareongdej S. Effect of Cha-em Thai mouthwash on salivary levels of mutans streptococci and total IgA. Southeast Asian J Trop Med Public Health. 2006 May;37(3):528-31. PMID: 17120974.
- Aneja KR, Joshi R, Sharma C. The antimicrobial potential of ten often used mouthwashes against four dental caries pathogens. Jundishapur J Microbiol. 2010; 3(1): 15-27.
- Avila M, Ojcius DM, Yilmaz O. The oral microbiota: living with a permanent guest. DNA Cell Biol. 2009 Aug;28(8):405-11. https://doi.org/10.1089/dna.2009.0874. PMID: 19485767; PMCID: PMC2768665.
- Axelsson P, Nyström B, Lindhe J. The long-term effect of a plaque control program on tooth mortality, caries and periodontal disease in adults. Results after 30 years of maintenance. J Clin Periodontol. 2004 Sep;31(9):749-57. https://doi.org/10.1111/j.1600-051X.2004.00563.x. PMID: 15312097.
- Barnett ML. The rationale for the daily use of an antimicrobial mouthrinse. J Am Dent Assoc. 2006 Nov;137 Suppl:16S-21S. https://doi.org/10.14219/jada.archive.2006.0408. Erratum in: J Am Dent Assoc. 2008 Mar;139(3):252. PMID: 17035671.
- Biswas S, Biswas I. Role of VltAB, an ABC transporter complex, in viologen tolerance in Streptococcus mutans. Antimicrobial Agents and Chemotherapy. 2011; 55 (4): 1460–9. https://doi.org/10.1128/AAC.0109410. PMC 3067168. PMID 21282456.
- Cummins D, Creeth JE. Delivery of antiplaque agents from dentifrices, gels, and mouthwashes. J Dent Res. 1992 Jul;71(7):1439-49. https://doi.org/10.1177/00220345920710071601. PMID: 1629461.
- Cummins D. Vehicles: how to deliver the goods. Periodontol 2000. 1997 Oct;15:84-99. https://doi.org/10.1111/j.1600-0757.1997.tb00108.x. PMID: 9643236.
- Dalirsani Z, Aghazadeh M, Adibpour M, Amirchaghmaghi M, Pakfetrat A, Mosannen Mozaffari P, Mehdipour M, Taghavi Zenooz A. In vitro Comparison of the Antimicrobial Activity of Ten Herbal Extracts Against Streptococcus mutans with Chlorhexidine. J Appl Sci. 2011; 11(5): 878-882. https://doi.org/10.3923/jas.2011.878.882
- Watanabe E, Tanomaru JM, Nascimento AP, Matoba-Júnior F, Tanomaru-Filho M, Yoko Ito I. Determination of the maximum inhibitory dilution of cetylpyridinium chloride-based mouthwashes against Staphylococcus aureus: an in vitro study. J Appl Oral Sci. 2008 Jul-Aug;16(4):275-9. https://doi.org/10.1590/s1678-77572008000400009. PMID: 19089260; PMCID: PMC4327537.
- Fernanda CR Lessa, Claudia HB Grillo, Fernanda E Pinto, Bethânia B Lorençon, João D L Martins, Suzan K V Bertolucci, José Eduardo B P Pinto, Denise C Endringer. Efficacy of guaco mouthwashes (Mikania glomerata and Mikania laevigata) on the disinfection of toothbrushes. Rev. Bras. Farmacogn. 2012 Dec; 22(6): 1330-1337. https://doi.org/10.1590/S0102-695X2012005000115
- Francetti L, del Fabbro M, Testori T, Weinstein RL. Chlorhexidine spray versus chlorhexidine mouthwash in the control of dental plaque after periodontal surgery. J Clin Periodontol. 2000 Jun;27(6):425-30. https://doi.org/10.1034/j.1600-051x.2000.027006425.x. PMID: 10883872.
- Van der Weijden FA, Van der Sluijs E, Ciancio SG, Slot DE. Can Chemical Mouthwash Agents Achieve Plaque/Gingivitis Control? Dent Clin North Am. 2015 Oct;59(4):799-829. https://doi.org/10.1016/j.cden.2015.06.002. PMID: 26427569.
- Furuichi Y, Ramberg P, Lindhe J, Nabi N, Gaffar A. Some effects of mouthrinses containing salifluor on de novo plaque formation and developing gingivitis. J Clin Periodontol. 1996 Aug;23(8):795-802. https://doi.org/10.1111/j.1600-051x.1996.tb00612.x. PMID: 8877668.
- Gibbons RJ, Houte JV. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19-44. https://doi.org/10.1146/annurev.mi.29.100175.000315. PMID: 1180512.
- Tal H, Rosenberg M. Estimation of dental plaque levels and gingival inflammation using a simple oral rinse technique. J Periodontol. 1990; 61(6): 339-342. https://doi.org/10.1902/jop.1990.61.6.339
- Hugoson A, Koch G, Johansson S (Eds). CONSENSUS Klorhexidin inom tandvården. Lic Forlag, Solna, 1990; 123.
- Jackson RJ. Metal salts, essential oils and phenols–old or new? Periodontol 2000. 1997 Oct;15:63-73. https://doi.org/10.1111/j.1600-0757.1997.tb00106.x. PMID: 9643234.
- Gunsolley JC. A meta-analysis of six-month studies of antiplaque and antigingivitis agents. J Am Dent Assoc. 2006 Dec;137(12):1649-57. https://doi.org/10.14219/jada.archive.2006.0110. PMID: 17138709.
- Karpiński TM, Szkaradkiewicz AK. Chlorhexidine-pharmaco-biological activity and application. Eur Rev Med Pharmacol Sci. 2015 Apr;19(7):1321-6. PMID: 25912596.
- Smith K, Robertson DP, Lappin DF, Ramage G. Commercial mouthwashes are ineffective against oral MRSA biofilms. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013 May;115(5):624-9. https://doi.org/doi: 10.1016/j.oooo.2012.12.014. Epub 2013 Mar 17. PMID: 23510687.
- Kigure T, Saito A, Seida K, Yamada S, Ishihara K, Okuda K. Distribution of Porphyromonas gingivalis and Treponema denticola in human subgingival plaque at different periodontal pocket depths examined by immunohistochemical methods. J Periodontal Res. 1995 Sep; 30(5): 332-41. https://doi.org/10.1111/j.1600-0765.1995.tb01284.x. PMID: 7494175.
- Loesche WJ. Chapter 99. Microbiology of Dental Decay and Periodontal Disease”. In Baron S; et al. Baron’s Medical Microbiology (4th ed.). University of Texas Medical Branch. 1996. ISBN 0963117211. PMID 21413316.
- Loe H, Theilade E, Jensen SB. 1965. Experimental gingivitis in man. J Periodontol (1930). 1965 May-Jun;36:177-87. https://doi.org/10.1902/jop.1965.36.3.177. PMID: 14296927.
- Liljemark WF, Bloomquist C. Human oral microbial ecology and dental caries and periodontal diseases. Crit Rev Oral Biol Med. 1996;7(2):180-98. https://doi.org/10.1177/10454411960070020601. PMID: 8875032.
- Madigan M, Martinko J. 2005. Brock Biology of Microorganisms (11th ed.). Prentice Hall. ISBN 0131443291.
- Mat Ludin CM, Md Radzi J. The antimicrobial activity of different mouthwashes in malaysia. Malays J Med Sci. 2001 Jul;8(2):14-8. PMID: 22893755; PMCID: PMC3413644.
- Bakhshi M, Taheri JB, Basir Shabestari S, Tanik A, Pahlevan R. Comparison of therapeutic effect of aqueous extract of garlic and nystatin mouthwash in denture stomatitis. Gerodontology. 2012; 29: e680-e684. https://doi.org/10.1111/j.1741-2358.2011.00544.x
- Marsh PD. 2000. Role of the Oral Microflora in Health, Microbial Ecology in Health and Disease. 2000; 12(3): 130-137. https://doi.org/10.1080/089106000750051800
- Marinho VC, Higgins JP, Logan S, Sheiham A. Fluoride mouthrinses for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 2003; 3: CD002284. https://doi.org/10.1002/14651858.CD002284. PMID: 12917928.
- Marya CM. A Textbook in Public Health Dentistry. JP Medical Ltd, 2011; 292–301.
- Majed M. Masadeha, C, Shadi F. Gharaibehb, Karem H. Alzoubib, Sayer I. Al-Azzamb, Wasfi M. Obeidata. Antimicrobial Activity of Common Mouthwash Solutions on Multidrug-Resistance Bacterial Biofilms. J Clin Med Res. 2013; 5(5):389-394.
- Meurman JH, Stamatova I. Probiotics: Contributions to oral health. Oral Diseases. 2007; 13 (5): 443–51. https://doi.org/10.1111/j.16010825.2007.01386. x. PMID 17714346.
- Moran JM. Chemical plaque control–prevention for the masses. Periodontol 2000. 1997 Oct;15:109-17. https://doi.org/10.1111/j.1600-0757.1997.tb00110.x. PMID: 9643238.
- Nattapon Sritrairat, Narin Nukul, Piyanut Inthasame, Attapon Sansuk, Jinda Prasirt, Thassin Leewatthanakorn, Uayporn Piamsawad, Aree Dejrudee, Pharkphoom Panichayupakaranant, Kanokporn Pangsomboon, Nilnara Chanowanna, Janpim Hintao, Rawee Teanpaisan, Wisut Chaethong, Pataraporn Yongstar, Nannapat Pruphetkaew, Virasakdi Chongsuvivatwong, Wipawee Nittayananta. Antifungal activity of lawsone methyl ether in comparison with chlorhexidine. J Oral Pathol Med. 2011; 40: 90–96. https://doi.org/10.1111/j.1600-0714.2010.00921.x
- Da Silva NB, Alexandria AK, De Lima AL, Claudino LV, De Oliveira Carneiro TF, Da Costa AC, Valença AM, Cavalcanti AL. In vitro antimicrobial activity of mouth washes and herbal products against dental biofilm-forming bacteria. Contemp Clin Dent. 2012 Jul;3(3):302-5. https://doi.org/10.4103/0976-237X.103623. PMID: 23293486; PMCID: PMC3532793.
- Mohamed NS, El-Zehery RR, Mourad MI, Grawish Mel-A. Impact of three different mouthwashes on the incidence of gingival overgrowth induced by cyclosporine-A: a randomized controlled experimental animal study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015 Sep;120(3):346-56. https://doi.org/10.1016/j.oooo.2015.05.005. Epub 2015 May 27. PMID: 26153120.
- Patters MR, Nalbandian J, Nichols FC, Niekrash CE, Kennedy JE, Kiel RA, Trummel CL. Effects of octenidine mouthrinse on plaque formation and gingivitis in humans. J Periodontal Res. 1986 Mar;21(2):154-62. https://doi.org/10.1111/j.1600-0765.1986.tb01447.x. PMID: 2937903.
- Philip D. Marsh. Role of the Oral Microflora in Health. Microbial ecology in health and disease. 2000; 12(3): 130-137. https://doi.org/10.1080/089106000750051800
- Bahna P, Hanna HA, Dvorak T, Vaporciyan A, Chambers M, Raad I. Antiseptic effect of a novel alcohol-free mouthwash: a convenient prophylactic alternative for high-risk patients. Oral Oncol. 2007 Feb;43(2):159-64. https://doi.org/10.1016/j.oraloncology.2006.02.002. Epub 2006 Jun 22. PMID: 16798063.
- Pourabbas R, Delazar A, Chitsaz MT. The effect of German chamomile mouthwash on dental plaque and gingival inflammation. Iranian J Pharma Res. 2005; 4(2): 105-9. https://doi.org/10.22037/ijpr.2010.624
- Palmer JrRJ , Chalmers N, Rickard A, Kolenbrander P. Community Development in Bacterial Biofilms of the Oral Cavity. Microscopy and Microanalysis. 2008;14(S2): 1554-1555. https://doi.org/10.1017/S1431927608088831
- Quintas V, Prada-López I, Donos N, Suárez-Quintanilla D, Tomás I. In situ neutralisation of the antibacterial effect of 0.2% Chlorhexidine on salivary microbiota: Quantification of substantivity. Arch Oral Biol. 2015 Aug;60(8):1109-16. https://doi.org/10.1016/j.archoralbio.2015.04.002. Epub 2015 Apr 24. PMID: 26022118.
- Rawlinson A, Pollington S, Walsh TF, Lamb DJ, Marlow I, Haywood J, Wright P. 2008. Efficacy of two alcohol-free cetylpyridinium chloride mouthwashes – a randomised double blind crossover study. J Clin Periodontol; 35: 230–235. https://doi.org/10.1111/j.1600-051X.2007.01187.x.
- Rogers A H. Molecular Oral Microbiology. Caister Academic Press. 2008; 24-0.ISBN 978-1-904455.
- Ryan KJ, Ray CG. Sherris Medical Microbiology (4th ed.). McGraw Hill. 2004. ISBN 0838585299
- Sbordone L, Bortolaia C. Oral microbial biofilms and plaque-related diseases: microbial communities and their role in the shift from oral health to disease. Clin Oral Investig. 2003 Dec;7(4):181-8. https://doi.org/10.1007/s00784-003-0236-1. Epub 2003 Nov 4. PMID: 14598129.
- Puig Silla M, Montiel Company JM, Almerich Silla JM. Use of chlorhexidine varnishes in preventing and treating periodontal disease. A review of the literature. Med Oral Patol Oral Cir Bucal. 2008 Apr 1;13(4):E257-60. PMID: 18379452.
- Santos Francisco AV, Serra Carlos G, Bezerra Roberto JAC, Figueredo Fernando G, Edinardo , Matias FF, Menezes Irwin RA, Costa Jos´e GM, Coutinho Henrique D.M. Antibacterial activity of Plectranthus amboinicus Lour (Lamiaceae) essential oil against Streptococcus mutans. Eur J Integr Med. 2016; 8(3): 293-297.
- Shahakbari R, Eshghpour M, Rajaei A, Rezaei NM, Golfakhrabadi P, Nejat A. Effectiveness of green tea mouthwash in comparison to chlorhexidine mouthwash in patients with acute pericoronitis: a randomized clinical trial. Int J Oral Maxillofac Surg. 2014 Nov;43(11):1394-8. https://doi.org/10.1016/j.ijom.2014.05.017. Epub 2014 Jun 18. PMID: 24954134.
- Suvan JE, D Aiuto F. Progressive, paralyzed, protected, perplexed? What are we doing?. International Journal of Dental Hygiene. 2008; 6: 251-252. https://doi.org/10.1111/j.1601-5037.2008.00347.x
- Twetman S, Stecksen Blicks C. Probiotics and oral health effects in children. Int J Paediatr Dent. 2008; 18 (1): 3–10. https://doi.org/10.1111/j.1365263X. 2007.00885.x. PMID 18086020.
- Weinberger B. 1948. Introduction to the history of dentistry. St Louis (MO): Mosby.
- Nittayananta W, Pangsomboon K, Panichayupakaranant P, Chanowanna N, Chelae S, Vuddhakul V, Sukhumungoon P, Pruphetkaew N. 2013. Effects of lawsone methyl ether mouthwash on oral Candida in HIV-infected subjects and subjects with denture stomatitis. J Oral Pathol Med. 2013; 42(9): 0904-2512. https://doi.org/10.1111/jop.12060 1-7.
ARTICLE TYPE: Research Article;
ORCID ID: Open Researcher and Contributor Identifier (ORCID) ID of corresponding author: https://orcid.org/0000-0002-4330-8158;
ETHICAL: NA; ACKNOWLEDGEMENT: None;
FINANCIAL DISCLOSURE: The authors declare that there was no financial aid received.;
CONFLICT OF INTEREST: No conflict of interest associated with this review work.;
AUTHORS CONTRIBUTION: Conceptualization: L.S.R.A., Supervision: D.N.P., Methodology: J.H.B., Writing: K.G.B.,;
CORRESPONDING AUTHOR AFFILIATIONS: Dr. Dhara N Patel, Department of Medical Laboratory Science, Bapubhai Desaibhai Patel Institute of Paramedical Science (BDPIPS), Charotar University of Science and Technology, Charusat Campus, Gujarat, India.;
CORRESPONDING AUTHOR EMAIL: dharapatel.cips@charusat.ac.in;
ARTICLE CITATION: RajA LS , Bera JH, Bhaine KG, Patel DN. Study on efficacy of commercially available mouth wash. SALT J Sci Res Healthc. 2021 Oct 30; 1(2):24-34.
PUBLISHER’S NOTE: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.




© Leyon Selvin Raj A, Jignasa H Bera, Kushani Girishbhai Bhaine and Dhara N Patel.
Originally published in the SALT Journal of Scientific Research in Healthcare (https://saltjsrh.in/), 29.10.2021.
This is an open-access article distributed under the terms of the Creative Commons License (https://creativecommons.org/licenses/by-nc-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the SALT Journal of Scientific Research in Healthcare (https://saltjsrh.in/), is properly cited. The complete bibliographic information, a link to the original publication on https://saltjsrh.in/, as well as this copyright and license information must be included.
