EVALUATION OF MODIFIED SEMI-MICRO METHOD FOR ESTIMATION OF GLUCOSE USING GOD POD ENDPOINT ASSAY
SALT Journal of Scientific Research in Healthcare
Volume 2, Issue 2, Page 05-10, Published on 05th October 2022
Research Article
SHERAFIN JANCY VINCY AND *M CHANDRASEKAR
Sree Balaji College of Physiotherapy,
Bharath Institute of Higher Education and Research, Chennai, India.
*Corresponding Author: Dr M Chandrasekar, Professor, Sree Balaji College of Physiotherapy, Bharath Institute of Higher Education and Research, Chennai, India
ORCID ID: 0000-0003-0290-3062
Email: shekarmlt@gmail.com
ABSTRACT
The present study evaluated a modified semi-micro (SMic) protocol with conventional macro (CMac) protocol for serum glucose level estimation using a semi-autoanalyser. Three hundred blood samples were collected from patients (ages 22 to 84) of Loyola Health Centre, Chennai. Each serum sample was aliquoted into two tubes, one for CMac protocol and the other for SMic protocol. Blood Glucose was estimated by GOD-POD endpoint assay for both methods using a semi-autoanalyser. Mann-Whitney U test revealed Kappa value 0.901 (se = 0.036) at 95% confidence interval for kappa = 0.831 to 0.971 and showed no significant differences (p < 0.0001) between two protocol. The SMic protocol reduces the test cost and provides an opportunity to screen many samples with fewer reagents. We conclude that SMic can be used as an alternative to CMac protocol to estimate serum glucose concentrations in diabetic patients.
Keywords: Serum glucose, diabetes mellitus, semi-auto analyser, SMic, CMac, GOD-POD.
INTRODUCTION
Glucose provides energy for life processes and is the primary end product of carbohydrate metabolism. Blood glucose is measured mainly for the diagnosis and management of diabetes mellitus. Reasonable control of blood glucose levels in diabetes helps to prevent or delay the development of complications, which may lead to premature disability or death from blindness, kidney failure, coronary thrombosis, stroke, bacterial infections, and fungal infections (Monica Cheesbrough, 1999). Diabetes mellitus (DM) is a chronic disorder and a global health issue. About 20 to 30 million people in India are diagnosed with DM. This figure is expected to rise to about 80 million by 2030 (Kaveeshwar and Cornwall, 2014; Wild et al., 2004). There is a need for continuous and stringent management of diabetic patients; maintaining the plasma glucose level within the acceptable range is essential for diabetic management. Various laboratory methods determine glucose levels (Basak, 2007). Although Glucometers are useful for self-monitoring and keeping records of glycemic control, they should be used with caution in emergency hypoglycemic conditions or cases of hyperglycemia as they can overestimate or under-estimate the glucose levels. In these conditions, it is advisable to determine and confirm the glucose concentration in the laboratory (Ayaz and Marya, 2017).
In laboratories so far, estimation of blood glucose levels is being done by the glucose oxidase/peroxidase method described by Trinder in 1969, using the conventional macro-protocol (CMac). This Trinder’s reagent is specific for glucose, readily available, stable and less of an occupational hazard than in other methods (Lott and Turner, 1975). However, the protocol involved a considerable amount of reagent and cost (Srikanth et al., 2004; Thomas, 1998). Consequently, a cheap yet reliable procedure for glucose estimation using the GOD/POD approach is required. In light of its importance, an attempt has been made in the present study to develop a new semi-micro-protocol (SMic) using a semi-auto analyser, which can provide an opportunity to reduce the cost of the test for patients.
MATERIALS AND METHODS
The present observational and cross sectional study was undertaken in the Loyola Health Centre. 300
blood samples altogether were obtained from healthy volunteers and diabetic patients between the ages of 22 and 84 who were willing to take part in the study.
Inclusion and exclusion criteria: All patients of either sex attending Loyola health centre with prior written consent were included in the study. Patients with major acute illness, recurrent myocardial infarction and who had refused to give informed consent were excluded from the study.
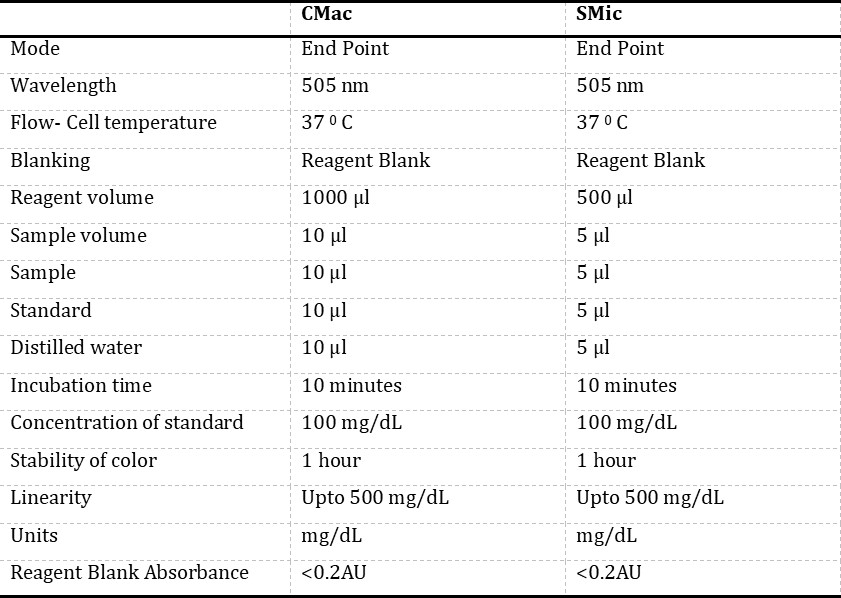
Equipment and reagents: Glucose estimation was done using the GOD-POD method based on endpoint assay using the kit of Span Diagnostics Limited, Surat. Each component of the kit was stable; it was kept firmly covered at 2-3° C, shielded from light and contamination while in use. Routinely signs of reagent deterioration, such as the presence of particles and turbidity and blank absorbance against the water, were checked. Semi-autoanalyser used in the study was Bayer’s Diagnostics, Model: RA – 50, and Micropipettes were of Microlit high precision pipette. The semi-auto analyser was programmed for endpoint assay as per the Span Diagnostics kit (Table 1).
Specimen collection: One milliliter of blood was drawn from the antecubital vein using sterile needles and a syringe provided by Becton Dickinson India Private Limited, New Delhi, with nearly aseptic precautions. Blood sample from each patient was collected into plain tubes and permitted to clot and stand uncentrifuged at room temperature, the average decrease in serum glucose is 7% 1 hour (5-10 mg/dl). The serum was separated from a non-hemolysed blood sample by centrifuging at 3500 rpm for 10 minutes using REMI research centrifuge. Glucose concentration is generally stable up to 8h at 250C or 72 hours at 40C in a separated non-hemolysed serum sample if kept free of bacterial contamination. Each serum sample was aliquoted for two tubes, one for CMac and the other for SMic protocol. Each tube was coded to reduce the bias. Glucose was estimated by GOD-POD endpoint assay for both methods using semi autoanalyser.
Principle of GOD-POD method: Beta-D-glucose is converted to gluconic acid by the enzyme glucose oxidase (GOD), which also releases hydrogen peroxide. Nascent oxygen is liberated when the peroxidase enzyme acts on hydrogen peroxide. The 4-amino antipyrine and phenol combine with nascent oxygen to create red quinone imine colour. The relationship between the colour’s intensity and the serum’s glucose level is straightforward. The intensity of the colour is measured colourimetrically at 530 nm and compared with that of a standard treated similarly (Trinder,1969; Sharma et al., 2017).
CMac protocol: The glucose reagent supplied along with the kit was aliquoted (1000 μl) into three different test tubes, and the tubes were marked as blank (B), test (T) and standard (S) (Table 1). An aliquot of 10μl of distilled water was added to the tube marked as “B”, later aliquots of 10μl of sample and 10μl of glucose standard (supplied with kit) were dispensed into the “T” and “S” tubes, respectively. The absorbance of the “T” was measured at 505 nm using a semi-auto analyser against a reagent “B”, and the concentration of glucose was noted.
SMic protocol: For SMic protocol method, the glucose reagent supplied along with the kit was aliquoted (500μl) into three different test tubes, and the tubes were marked as blank (B), test (T) and standard (S) (Table 2). An aliquot of 5μl of distilled water was added to the tube marked as “B”, later aliquots of 5μl of sample and 5μl of glucose standard (supplied with kit) were dispensed into the “T” and “S” tubes, respectively. The absorbance of the test was measured at 505 nm using a semi-auto analyser against a reagent “B”, and the concentration of glucose was noted.
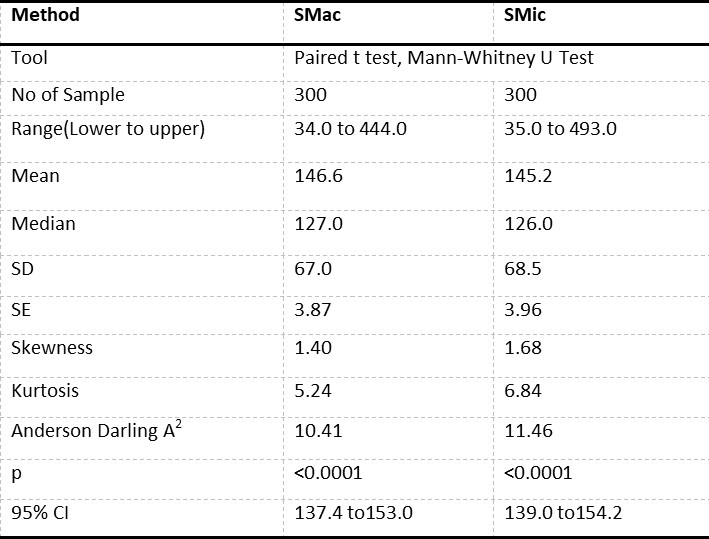
Statistical analysis: The results obtained through CMac and SMic protocol was recorded on Microsoft Excel and were subjected to statistical analysis by calculating the range, mean (M), median, standard deviation (SD), standard error (SE), skewness, kurtosis, Anderson darling A2, p-value and K value by paired t-test and Mann Whitney-U test (Table 2).
RESULTS AND DISCUSSION
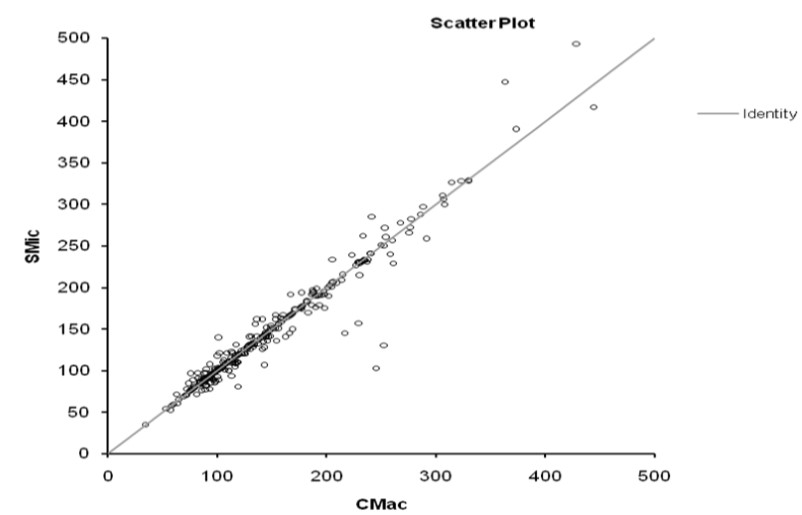
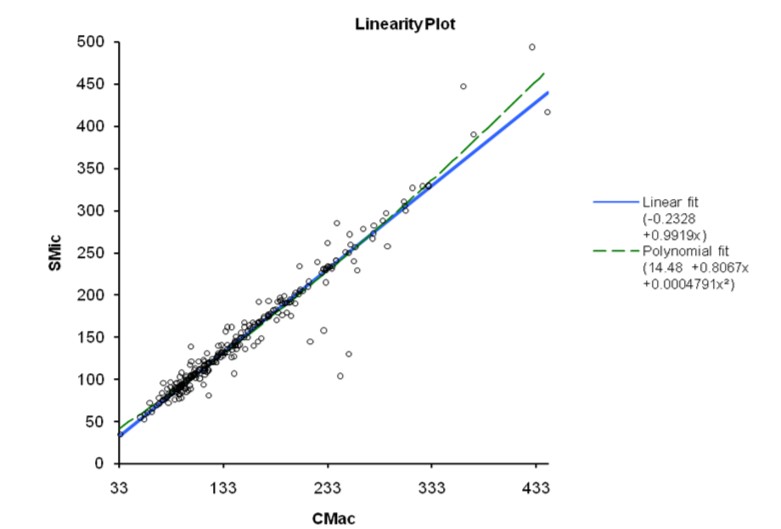
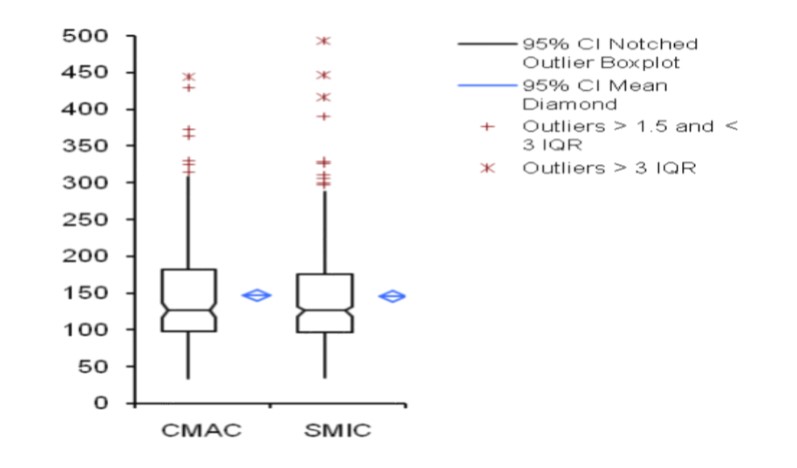
The results obtained from 300 patients by SMic protocol using the semi-autoanalyzer have yielded almost similar values as that of CMac protocol, with a slight variation (Table 2). The results envisaged that the calculated M was 146.6 and 145.2, SD were 67.0 and 68.5, and SE were 3.87 and 3.96 for CMac and SMic protocol, respectively (figure 3). Both protocols were correlated and depicted graphically in figure 1. Mann-Whitney U test revealed Kappa value 0.901 (se = 0.036) at 95% confidence interval for kappa = 0.831 to 0.971 and showed no significant differences (P < 0.0001) between two protocols. Lower ranges of glucose estimation were 34.0 & 35.0 mg/dL and upper range 444.0 & 493.0 mg/dL for CMac & SMic protocol respectively (Table 2). The upper range can be taken up to 500 mg/dL (Table 1); the linearity of CMac & SMic protocol is depicted in figure 2.
In India, diabetes mellitus is considered a disease of grave concern not only because of the rapidly increasing prevalence of this disease but also because various studies have shown the rising prevalence of diabetes in young and middle-aged persons. Increased diabetes mellitus would also increase the disease burden and put socioeconomic pressure on the country’s most productive age group and health systems (Mohan et al., 2007).
Inaccurate laboratory glucose results can misidentify patients as having diabetes. Failure to diagnose leads to serious long-term consequences because diabetes carries an increased risk of cardiovascular disease, and its treatment can delay or prevent the onset of type 2 diabetes (Knowler et al., 2002).
The fundamental role of health care laboratories is to generate reliable data for time and cost-efficient clinical decision-making (Njab et al., 2005). Not only must people with diabetes monitor their glucose concentration and take action to keep these values within acceptable limits (Hicks, 1985). Glucometer, though it gives a rapid result of blood glucose estimation, is not recommended because of its potential for imprecision (Sacks et al., 2002; Basak, 2007).
Plasma glucose estimation based on the Glucose oxidase – peroxidase (GOD-POD) method for the colourimetric determination was first introduced by Worthington in 1956. Furthermore, it was improved by other individuals, including Trinder (Trinder, 1969), and it quickly gained popularity in place of the lengthy and time-consuming multistep chemical methods for glucose quantification.
The GOD-POD method is linear (up to 500 mg/dl), sensitive (detection limit 0.3 mg/dl), simple (requires 10 µL of sample to be incubated for 30 minutes with a single reagent at room temperature) and requires simple instrumentation (the absorbance to be read between 505 nm to 550 nm) (Ambade et al.,1998). CMac protocol uses 1ml of glucose reagent for one test in endpoint chemistry and costs around 40-50 Rs; SMic protocol reduces each test’s cost to half, giving ultimate benefit to patients. Hence, the new SMic protocol was evaluated.
The SMic protocol for serum glucose estimation using a semi-auto analyser can provide an opportunity to detect glucose levels very effectively with minimum consumption of reagents. SMic has an excellent correlation with the CMac protocol. With either protocol, the method is suitable for analysing samples with glucose concentrations of <500 mg/dL. If the value exceeds the linearity limit, the sample can be diluted with normal saline (0.85% NaCl), and the results can be multiplied with the dilution factor to obtain the final result.
Based on the observations in the present study, the use of a new SMic protocol is recommended for serum glucose estimation, mainly when there is a need for repeated or regular quantification of glucose levels in severe diabetic conditions, as it provides an opportunity to screen more number of samples with the same amount of reagent. GOD-POD method is not influenced by uric acid, ascorbic acid, glutathione, bilirubin and creatinine at physiological concentration.
The limitation of the study is that quality control serums were not used. According to Span Diagnostics Ltd.’s instructions, the reagent may over time take on a pale pink hue, however this does not impair its effectiveness up to reagent blank absorbance of 0.20 AU. SMic protocol shall be evaluated with other biochemical parameters, and feasibility with calorimeter shall be assessed.
CONCLUSION
Considering the precision, reproducibility and cost-effectiveness of SMic protocol, it could be conveniently adopted to determine blood glucose levels.
REFERENCES
- Basak A. Development of a rapid and inexpensive plasma glucose estimation by two-point kinetic method based on glucose oxidase-peroxidase enzymes. Indian J Clin Biochem. 2007 Mar; 22(1):156-60. https://doi.org/10.1007/BF02912902. PMID: 23105673; PMCID: PMC3454249.
- Mallick AK, Ahsan M, A comparative study of glucose concentration determined from venous plasma sample and capillary blood sample. Int J Clin Biochem Res. 2017;4(3):220-224. https://doi.org/10.18231/2394-6377.2017.0052
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002 Feb 7;346(6):393-403. https://doi.org/10.1056/NEJMoa012512. PMID: 11832527; PMCID: PMC1370926.
- Hicks JM. In situ monitoring. Clin. Chem. 1985; 31(12): 1931-1935.
- Lott JA, Turner K. Evaluation of Trinder’s glucose oxidase method for measuring glucose in serum and urine. Clin Chem. 1975 Nov;21(12):1754-60. PMID: 1237363.
- Kaveeshwar SA, Cornwall J. The current state of diabetes mellitus in India. Australas Med J. 2014 Jan 31;7(1):45-8. https://doi.org/10.4066/AMJ.2013.1979. PMID: 24567766; PMCID: PMC3920109.
- Mohan V, Sandeep S, Deepa R, Shah B, Varghese C. Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res. 2007 Mar; 125(3):217-30. PMID: 17496352.
- Monica Cheesbrough. District Laboratory Practice in Tropical Countries, Part 1, Cambridge University Press. 1999; 340-346.
- Njab J E, Salifu RO, Ezenwelu FU, Abdullai JJ, Oriaku D, and Enuma J. The quality of glucose testing in Abuja Metropolis. J Med Lab Science 2005; 14 (2): 23-28. https://doi.org/10.4314/jmls.v14i2.35324
- Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2002 Mar; 48(3):436-72. PMID: 11861436.
- Srikanth M, Rao GV, Rao KR. Modified assay procedure for the estimation of serum glucose using microwell reader. Indian J Clin Biochem. 2004 Jan; 19(1):34-5. https://doi.org/10.1007/BF02872385. PMID: 23105422; PMCID: PMC3453919.
- Sharma S P, Anjankar A P, Kale A, Comparison of glucose levels using glucometer and GOD-POD Method in diabetic patients. Int J Clin Biochem Res. 2017; 4(1): 6-10.
- Thomas L. Clinical Laboratory Diagnostics, 1st edition, TH- books Veriagsgseller Chaft. 1998; 980- 865.
- Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen receptor, Anal Clin Biochem 1969; (6): 24-27.
- Ambade VN, Sharma YV, Somani BL. Methods for estimation of blood glucose: a comparative evaluation. Med J Armed Forces India. 1998 Apr; 54(2):131-133. https://doi.org/10.1016/S0377-1237(17)30502-6. Epub 2017 Jun 26. PMID: 28775446; PMCID: PMC5531325.
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004 May; 27(5):1047-53. https://doi.org/10.2337/diacare.27.5.1047. PMID: 15111519.
ARTICLE TYPE: Research Article;
ORCID ID: Open Researcher and Contributor Identifier (ORCID) ID of corresponding author: https://orcid.org/0000-0003-0290-3062;
ETHICAL: Permission from Institutional Ethical Committee;
ACKNOWLEDGEMENT: We gratefully acknowledge the encouragement from The Management, Loyola College, Chennai and The Head, Department of Advanced Zoology and Biotechnology and Coordinator, Loyola Health Centre, Loyola College, Chennai.;
FINANCIAL DISCLOSURE: The authors declare that there was no financial aid received.;
CONFLICT OF INTEREST: No conflict of interest associated with this research work.;
AUTHORS CONTRIBUTION: M.C., Performed the experiments, S.J.V., Designed research. Both authors discussed the results and prepared manuscript.; AUTHOR AFFILIATIONS: Dr Sherafin Jancy Vincy, Professor, Sree Balaji College of Physiotherapy, Bharath Institute of Higher Education and Research, Chennai, India; Dr M. Chandrasekar, Professor, Sree Balaji College of Physiotherapy, Bharath Institute of Higher Education and Research, Chennai, India;
CORRESPONDING AUTHOR EMAIL: drsjvincy@gmail.com;
ARTICLE CITATION: Vincy SJ, Chandrasekar M. Evaluation of modified semi-micro method for estimation of glucose using GOD POD endpoint assay. SALT J Sci Res Healthc. 2022 October 05; 2(2): 05-10.
PUBLISHER’S NOTE: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.



© Sherafin Jancy Vincy and M Chandrasekar.
Originally published in the SALT Journal of Scientific Research in Healthcare (https://saltjsrh.in/), 05.10.2022.
This is an open-access article distributed under the terms of the Creative Commons License (https://creativecommons.org/licenses/by-nc-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the SALT Journal of Scientific Research in Healthcare (https://saltjsrh.in/), is properly cited. The complete bibliographic information, a link to the original publication on https://saltjsrh.in/, as well as this copyright and license information must be included.
